Commercially Importance Of Alcohols : Ethanol and pure or denatured alcohol have proven indispensable in the manufacturing of skin care products, detergents, hand sanitizers, and other industrial items, depending on the type of application. Since 2020, the pharmaceutical and cosmetic industries have seen a sharp increase in demand for industrial alcohol.
Alcohol-derived compounds are utilized in many different fields and daily life. Let's examine these substances in more detail. Alcohol used for commercial purposes is referred to as commercial alcohol. The most widely used commercial alcohols are followings:
1. Methanol
2. Ethanol
3. Isopropyl Alcohol
4. Ethylene glycol
5. Glycerol
Methanol
Methanol: The original method of producing methanol (methyl alcohol) involved heating wood chips without the presence of air. Methanol is produced when some of the carbohydrates in the wood are broken down; the methanol vapor is then condensed. As a result of this procedure, methanol is now also known by the name wood alcohol. Commercial methanol is produced by a catalytic reaction between hydrogen gas (H 2 ) and carbon monoxide (CO) at high pressure and temperature.
CO + 2H
2
CH
3
OH
Synthesis gas methanol
Coal can be partially burned in the presence of water to produce the mixture of hydrogen and carbon monoxide needed to produce methanol. The right ratio of hydrogen to carbon monoxide can be achieved by carefully controlling the amount of water added.
3C + 4H
2
O
CO
2
+ 2CO + 4H
2
Methanol’s Uses
-
Methanol is a popular industrial solvent because of its superior polar organic solvent qualities. But if significant amounts are breathed or consumed, it is more poisonous than ethanol and can result in blindness or even death.
-
For denaturing ethyl alcohol, i.e. to make it unfit for drinking purposes. Denatured alcohol is called methylated spirit.
-
Methanol is an excellent fuel for car engines because of its high-octane rating and low pollution emissions.
-
In the manufacturing of formaldehyde, perfumes and drugs.
Ethanol
Ethanol: Since ancient times, people have made ethanol (also known as ethyl alcohol), primarily by fermenting fruit juices. This unrefined wine was safe to drink all winter long because the fermented juice could be kept in an airtight container. Ethanol is known as grain alcohol because it is frequently made from grains like corn (maize), wheat, rye, and barley.
Fermentation process
1. Wheat, barley, and potato are common materials for these chemical equilibrium reactions.
2. For one hour, the mashed materials are cooked at 50°C. The melt contains the enzyme diastase, which hydrolyzes starch to convert maltose and sugar.
2(C 6 H 10 O 5 ) n + nH 2 O → n C 12 H 22 O 11
3. The liquid is cooled to 300°C and fermented for 1 to 3 days with yeast. Maltase is an enzyme found in yeast that converts maltose to glucose.
4. Zymase further converted glucose into ethanol.
As a byproduct, the carbon dioxide gas is recovered and sold. Fermentation produces a solution that is only about 12-15 percent alcohol because higher concentrations are toxic to yeast cells. This solution, however, can be distilled to increase the ethanol content to as much as 95%. The process of fermentation is comparatively costly when producing ethanol. The most popular method for producing industrial ethanol is the high-temperature catalytic addition of water to ethylene (C 2 H 4 ).
Ethanol’s Uses
-
Ethanol is a very good solvent for paints, lacquers, varnishes, dyes, cosmetics, perfumes, tinctures, cough syrups etc.
-
As power alcohol a mixture of 20% absolute alcohol and 80% petrol (gasoline) with benzene or tetralin as a cosolvent.
-
As an important starting material for manufacture of ether, chloroform, Iodoform etc.
-
As an antifreeze in automobile radiators.
Isopropyl alcohol
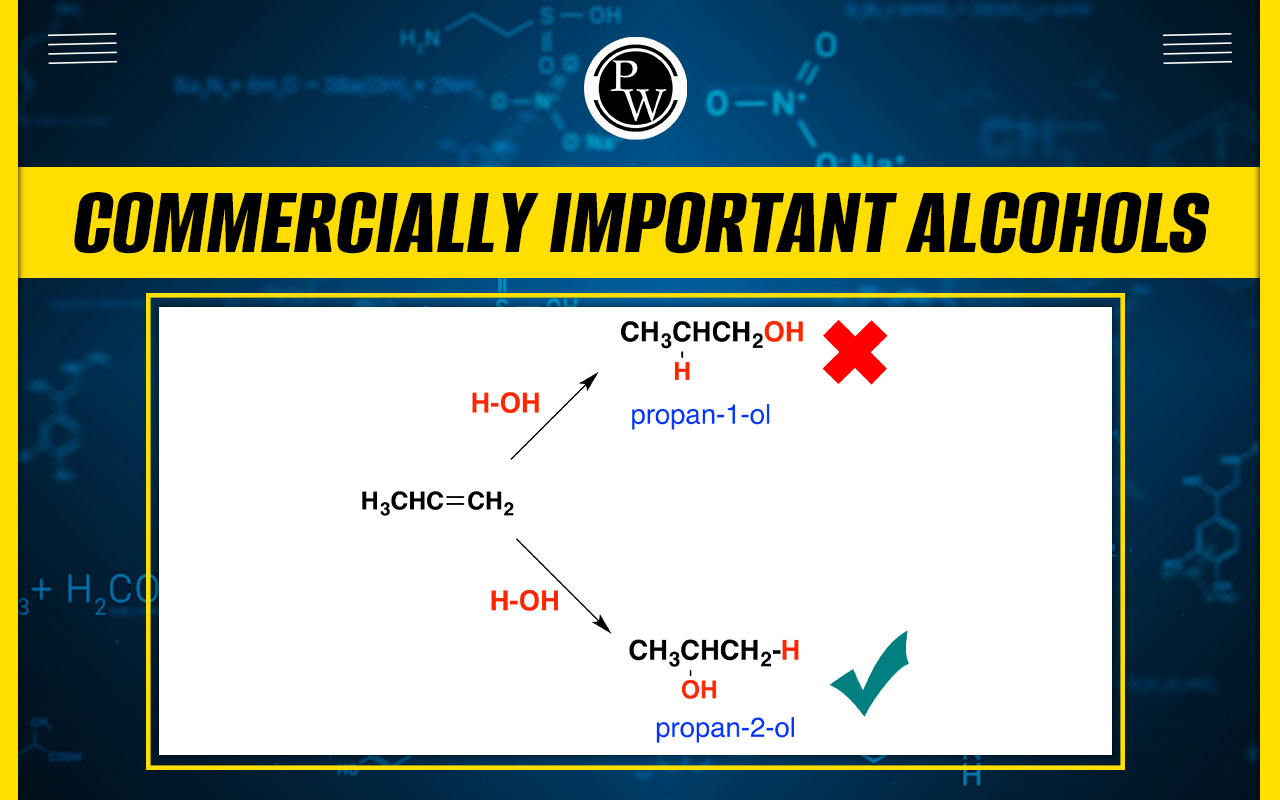
Isopropyl alcohol : Isopropanol, also known as isopropyl alcohol, is a clear, colorless liquid that is a major component of rubbing alcohol and other common household items such as cleaners, disinfectants, and hand sanitizers; it is also found in pharmaceuticals. Isopropyl alcohol (2-propanol) is synthesized through the indirect hydration of propylene (CH 2 CHCH 3 ).
Isopropyl Alcohol Uses
-
Isopropyl alcohol is commonly used as an industrial solvent and as a skin rubbing alcohol.
-
Using rubbing alcohol with a concentration of 90% or higher can result in a fast-evaporating cleaner for your electronics. Clean your computer's keyboard and mouse with an alcohol-soaked cotton swab or a damp alcohol-soaked microfiber cloth.
Ethylene Glycol
Ethylene Glycol : The organic compound ethylene glycol has the formula (CH 2 OH) 2 . It is primarily used as a raw material in the production of polyester fibers and in antifreeze formulations. It is a viscous, odorless, colorless, flammable liquid. It has a sweet taste, but in high concentrations it is toxic. The name ethylene glycol literally means "the glycol made from ethylene." Its formal name is ethane-1,2-diol.
Ethylene glycol’s Uses
-
The major use of ethylene glycol is as an antifreeze agent in the coolant.
-
It is used as a raw material in the manufacture of polyester fibers.
Glycerol
Glycerol: Glycerol is an alcohol that occurs naturally. It is a colorless liquid that is used as a solvent, sweetener, and medicine. Glycerol (also known as glycerin) is a sweet, syrupy substance that contains three hydroxyl groups from alcohol. It is known scientifically as
propane-1,2,3-triol. Glycerol was first produced as a byproduct of soap production, via the saponification (hydrolysis in base) of fats. Glycerol can be prepared synthetically from propylene (CH 2 =CH―CH 3 ).
Glycerol’s Uses
-
The main explosive in dynamite and gelatin blasting is nitroglycerin, which is made from glycerol.
-
The applications of glycerol include water-soluble lubricant, plasticizer, solvent, moisturizing agent, and antifreeze. Many different products contain it, such as foods, cosmetics, soaps, printing inks, hydraulic fluids, and medications.
-
Nitroglycerin is also used as a coronary vasodilator (a drug that relaxes and expands blood vessels) to treat chest pain caused by poor heart circulation.
Commercially importance Of alcohols FAQs
Q.1 : What are commercially importance of alcohols?
Q.2 : What is methylated spirit?
Q.3 : What is Glycerol?