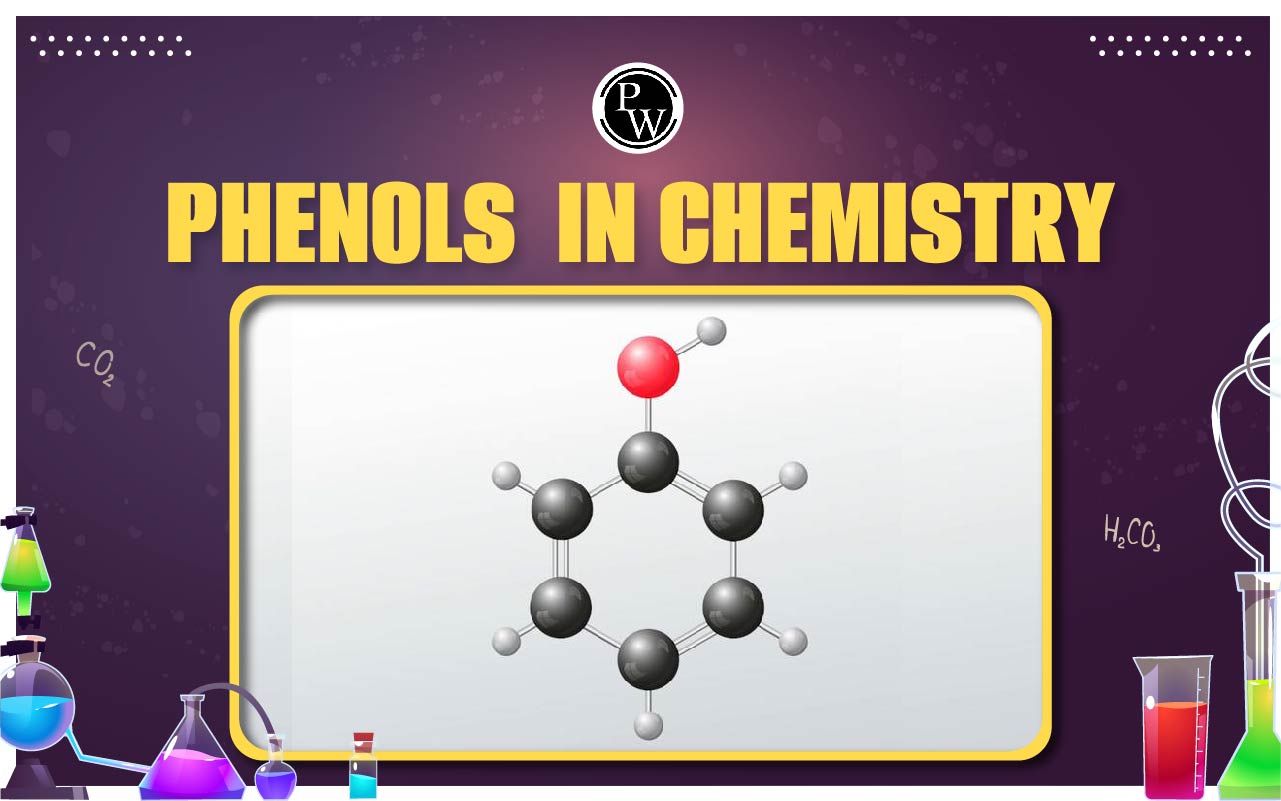
Phenols : Any member of the phenol family of organic compounds is identified by the presence of an aromatic ring-attached hydroxyl (OH) group to a carbon atom. Phenol, or carbolic acid, is the simplest member of the family and is referred to specifically as monohydroxy benzene (C 6 H 5 OH). The term phenol also serves as the generic name for the entire family.
Alcohols and phenols are similar, but phenols create stronger hydrogen bonds. As a result, they have higher boiling points and are more soluble in water than alcohols. At room temperature, phenols can be either colorless liquids or white solids. They can also be extremely caustic and toxic. Organic compounds that have at least one hydroxyl group linked to either an aryl or a saturated carbon are known as phenols and alcohols, respectively.
A related third class of compounds are called enols, because they have a vinylic carbon bonded to a hydroxyl group.
Phenols find extensive application in household products and as industrial synthesis intermediates. For instance, mouthwash and household cleansers both use phenol (in small amounts) as a disinfectant. It's possible that phenol was the original surgical antiseptic. Phenol is highly toxic, and concentrated solutions cause severe but painless burns to the skin and mucous membranes.
Phenol is a starting material used in industry to make plastics, drugs like aspirin, and explosives like picric acid. In photographic development, exposed silver bromide crystals are reduced to black metallic silver by a common ingredient called phenol hydroquinone.
[caption id="" align="alignleft" width="77"]
Structure of Phenol
Structure of Phenol : Every carbon atom in an aromatic ring has undergone sp2 hybridization. As a result, the structure of phenyl is hexagonal planar, with delocalized π-electrons dispersed throughout the ring and all bond angles at 120°. O-H bonds are formed from Osp 3 -H 1s , and C-O bonds are formed from Csp 2 -Osp 3 .
Two nonbonded electron pairs occupy two of the oxygen atom's other orbitals. Because of this, the hydroxyl functional group C-O-H, as shown in Figure below, has a bent shape and nearly a tetrahedral bond angle of 109.5°. Because oxygen has a greater electronegative potential than both carbon and hydrogen, both C-O and O-H bonds are polar.
Classification of Phenols
Classification of Phenols: Phenols are classified into three types based on the number of hydroxyl groups they contain.
- Monohydric phenols : They contain one -OH group.
- Dihydric phenols : They contain two -OH groups. They may be ortho-, meta- or para- derivative
- Trihydric phenols : They contain three -OH groups.
| Classification of Phenols | |
| Monohydric Phenol |
Monohydric Phenol
|
| Dihydric Phenol |
|
| Trihydric Phenol |
Trihydric Phenol
|
| Substituted Phenol |
Substituted Phenol
|
Phenol FAQs