Structure of Atom : The Structure of Atom is a fundamental concept in chemistry. It includes the arrangement of subatomic particles within an atom. At its core lies the nucleus, made up of protons (positively charged) and neutrons (neutral). Electrons, negatively charged particles, orbit around the nucleus.
Democritus, an ancient philosopher, first proposed the idea that matter consists of indivisible atoms. In the late 19th and early 20th centuries, scientists discovered subatomic particles, leading to the development of modern atomic models. Understanding atomic structure is crucial for explaining chemical properties and reactions.
Structure of Atom -Discovery of Electron (Cathode Rays)
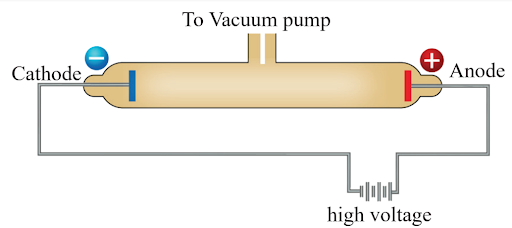
During the late 19th century, scientists were investigating cathode rays, which were mysterious streams of particles emitted from cathodes (negatively charged electrodes) in vacuum tubes. They sought to understand the nature of these rays and their role in the matter. In 1897, J.J. Thomson, an English physicist, designed a glass tube that was partially evacuated (all air removed). He applied a high electric voltage across the tube between two electrodes: the cathode (negative) and the anode (positive). Thomson observed a particle stream (ray) emerging from the cathode and moving toward the anode. This ray, known as the cathode ray, exhibited unique properties. Thomson discovered that the mass of these particles was much lighter than hydrogen, the lightest element. These subatomic particles came to be known as electrons. Thomson’s groundbreaking work laid the foundation for our understanding of the atomic structure and the existence of negatively charged particles within atoms. The discovery of electrons revolutionized physics and paved the way for further advancements in particle physics and quantum mechanicsProperties of Cathode Rays
- Origin of Cathode Rays: Cathode rays are streams of electrons emitted from the cathode (negative electrode) in a vacuum tube when it is subjected to high voltage.
- J.J. Thomson’s discover the electron in his experiments with cathode rays
- Cathode rays create shadows and move in straight directions..
- They can be deflected by electric and magnetic fields, indicating their charge (negative).
- They possess kinetic energy and momentum.
- Cathode rays move at high speeds, typically around 1/10th the speed of light.
- Their energy depends on the accelerating voltage applied to the cathode.
- Cathode rays consist of electrons (negatively charged particles).
- These electrons are fundamental constituents of matter.
- When cathode rays strike a fluorescent screen, they cause it to emit visible light.
- This property helped in the development of cathode ray tubes (CRTs) for television and monitors.
- Cathode rays can heat materials upon impact due to their kinetic energy.
- This phenomenon is utilized in electron microscopes and X-ray tubes.
- Their energy depends on the accelerating voltage applied to the cathode.
- Cathode rays consist of electrons (negatively charged particles).
- These electrons are fundamental constituents of matter.
- When cathode rays strike a fluorescent screen, they cause it to emit visible light.
- This property helped in the development of cathode ray tubes (CRTs) for television and monitors.
- Cathode rays can heat materials upon impact due to their kinetic energy.
- This phenomenon is utilized in electron microscopes and X-ray tubes.
Charge to Mass Ratio of Electron
The charge-to-mass ratio of an electron, denoted as e/m, is approximately 1.76 × 10^11 C/kg. This ratio represents the relationship between the charge of an electron (e) and its mass (m). J.J. Thomson’s experiments with cathode rays led to this groundbreaking discovery, revolutionizing our understanding of atomic structure and subatomic particles.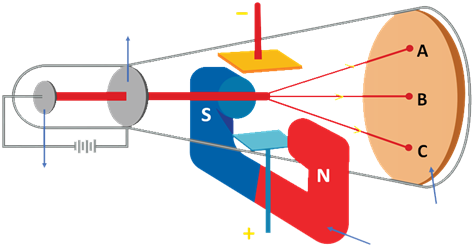
Charge on The Electron
An electron is a subatomic particle with an elementary charge of magnitude -1.602 × 10^-19 coulomb. It has a mass of approximately 1/1837 that of a proton. Discovered by J.J. Thomson in 1897, electrons orbit the nucleus in atomic orbitals.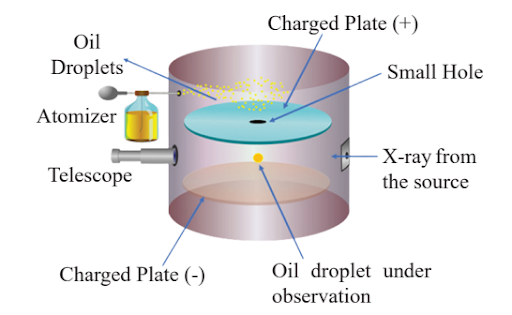
Structure of Atom - Discovery of Protons (Anode Rays)
Protons are positively charged fundamental subatomic particles found in the nucleus of atoms. In 1886, German physicist Eugen Goldstein conducted the Canal Ray Experiment using a modified cathode ray tube. In this experiment, he observed positively charged rays known as canal rays or anode rays. These rays originated from the anode (the positive electrode) and traveled in straight lines. The charge on these particles depended on the number of electrons lost by atoms. Protons play a crucial role in forming the nucleus of an atom and were first described by Ernest Rutherford in 1920.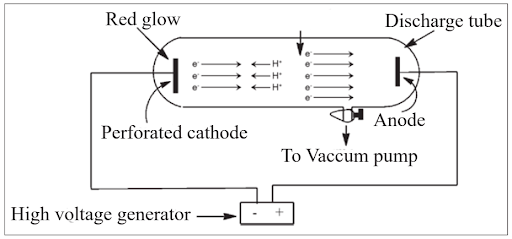
Properties of Anode Rays
Straight Trajectory : Anode rays travel in a straight line. Positive Charge : They carry a positive charge and are deflected toward the negative plate. Origin : These rays originate from the anode. Particle Charge : The charge on anode ray particles depends on the number of electrons lost by atoms
Structure of Atom - Discovery of Neutrons
The discovery of neutrons is credited to the British physicist James Chadwick in 1932. He was awarded the Nobel Prize in Physics for this groundbreaking discovery in 1935. Before this, scientists assumed that atoms consisted only of protons and electrons. Chadwick’s work revealed the existence of uncharged neutrons, fundamentally shaping our understanding of atomic structure.Thomson Model of Atom
It was proposed by J.J. Thomson in 1904, this model (also known as the Plum Pudding Model) suggested that atoms consist of a positively charged sphere with negatively charged electrons embedded within it. However, it failed to explain the stability of an atom and the position of the nucleus.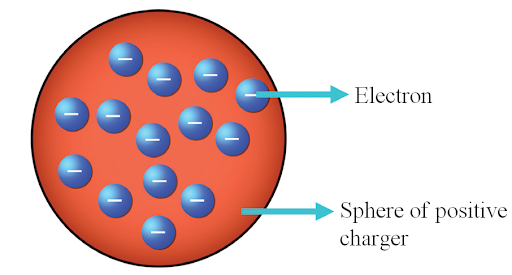 RADIOACTIVITY
Radioactivity is the property of certain matter to spontaneously emit energy and subatomic particles. Unstable atomic nuclei undergo decay, emitting particles or electromagnetic energy. It’s expressed in terms of half-life, ranging from extremely long to incredibly short timescales.
RADIOACTIVITY
Radioactivity is the property of certain matter to spontaneously emit energy and subatomic particles. Unstable atomic nuclei undergo decay, emitting particles or electromagnetic energy. It’s expressed in terms of half-life, ranging from extremely long to incredibly short timescales.
Rutherford’s Alpha Particle Experiment
Ernest Rutherford exposed a thin piece of gold foil to an alpha particle bombardment in 1911. His observations contradicted Thomson’s model: most of the atom is space, positive charge isn’t uniformly distributed, and the nucleus is a tiny, dense region. Rutherford postulated that electrons travel in a circular orbit around the nucleus.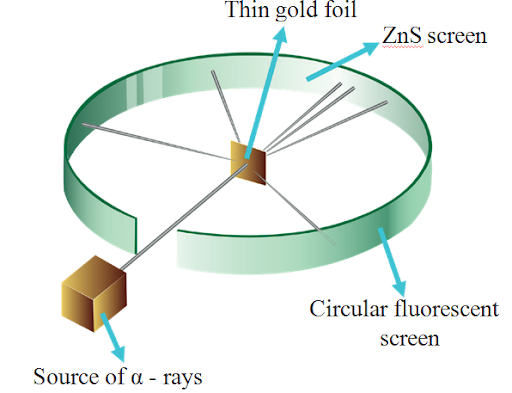 Rutherford’s Observations
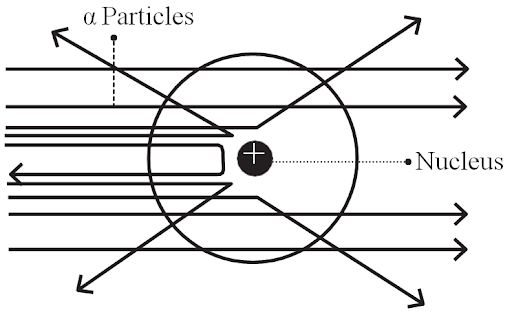
Rutherford’s Observations
- Most of the α-particles (nearly 99%) passed through the gold foil undeflected.
- A portion of the α-particles experienced slight angle deflection.
- Very few α -particles (1 in 20000) were either deflected by very large angles or were reflected along their path.
Drawbacks of the Rutherford Model
Electron Stabilit y - According to Rutherford, electrons revolve around the nucleus in circular paths. However, this motion would cause acceleration and energy radiation, leading to electron energy loss and instability. Lack of Explanation - The model failed to explain the stable arrangement of electrons around the nucleusAtomic Number
A crucial characteristic of an atom is its atomic number (Z). It shows how many protons there are in an atom's nucleus. Each chemical element has a unique atomic number, which determines its position in the periodic table. For example, hydrogen (H) has an atomic number of 1, indicating one proton in its nucleus.Mass Number
The total number of nucleons in an atom can be determined using the mass number (A).It represents the total of all the protons and neutrons that are found in the nucleus. Unlike the atomic number, which is specific to each element, the mass number can vary within the same element due to the existence of isotopes. For example, carbon-12 (12C) has a mass number of 12, indicating 6 protons and 6 neutrons.Isotopes
Isotopes are variants of an element with the same atomic number (same number of protons) but different numbers of neutrons. These variations occur naturally and result in atoms with slightly different masses. Isotopes exhibit identical chemical behavior due to their shared number of protons, but their nuclear composition differs. For example, carbon-14 (14C) has 6 protons like carbon-12 (12C), but it contains 8 neutrons.Isobars
Isobars are atoms of different chemical elements that share the same mass number (total nucleon count) but have distinct atomic numbers. In other words, their total number of protons and neutrons is identical. For example, argon-40 (40Ar), potassium-40 (40K), and calcium-40 (40Ca) are isobars, all having a mass number of 40 but different atomic numbers. Isotones Isotones are nuclides (nuclei or atoms) that share the same number of neutrons (N) but have different numbers of protons (Z). Unlike isotopes, which have the same atomic number, isotones focus on neutron count. For example, sulfur-36 (36S), chlorine-37 (37Cl), and potassium-39 (39K) are isotones. They all contain 20 neutrons but vary in their atomic numbers.
Maxwell Wave Theory
Maxwell’s Electromagnetic Wave Theory describes the relationship between changing electric and magnetic fields, leading to the propagation of electromagnetic waves (or light waves). These waves don’t require a material medium for transmission and travel at the speed of light. Maxwell’s equations form the foundation of classical electromagnetism and optics, revealing a spectrum of invisible waves beyond visible light.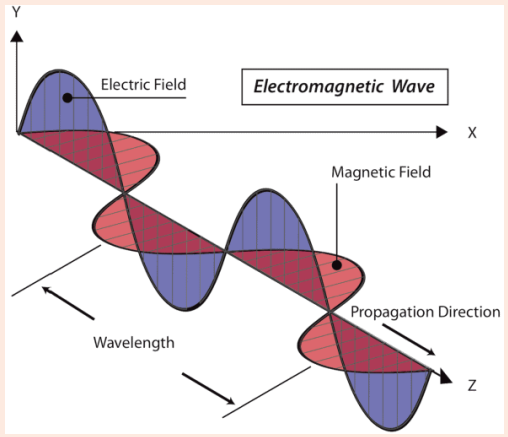
Properties of Electromagnetic Waves
Transverse Nature - Electromagnetic waves are transverse, meaning their oscillations occur perpendicular to the direction of wave propagation. Self-Sustaining Oscillations - These waves consist of electric and magnetic fields oscillating in space, with no need for a material medium. Speed of Light - Electromagnetic waves travel at a constant velocity of approximately 3 x 10^8 meters per second in a vacuum. Equation - They follow the equation c = fλ, where c is the speed of light, f is the frequency (in Hertz), and λ is the wavelength (in meters). Phase Relationship - The oscillating electric and magnetic fields are in the same phase, with their amplitudes related to the wave’s velocity.Characteristics of Electromagnetic waves
Below is the graph which shows the Characteristics of Electromagnetic waves. With this sinusoidal wave, the characteristics of electromagnetic waves can be easily understood.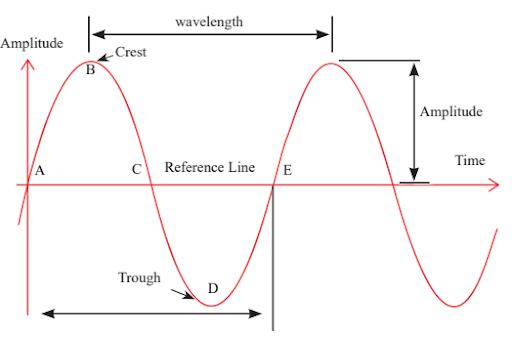 Electromagnetic waves Important Terminologies
Wavelength
: Distance between successive wave crests or troughs. Represents the wave’s spatial extent.
Frequency
: Number of oscillations (cycles) per second. Measured in Hertz (Hz).
Velocity
: Speed at which a wave propagates. For light, it’s approximately 3 x 10^8 m/s.
Wave Number
: Reciprocal of wavelength. Denoted by k. Relates to spatial frequency.
Amplitude
: Maximum displacement from equilibrium. Determines wave’s intensity or energy.
Time Period
: Duration for one complete oscillation. Inversely related to frequency.
Electromagnetic waves Important Terminologies
Wavelength
: Distance between successive wave crests or troughs. Represents the wave’s spatial extent.
Frequency
: Number of oscillations (cycles) per second. Measured in Hertz (Hz).
Velocity
: Speed at which a wave propagates. For light, it’s approximately 3 x 10^8 m/s.
Wave Number
: Reciprocal of wavelength. Denoted by k. Relates to spatial frequency.
Amplitude
: Maximum displacement from equilibrium. Determines wave’s intensity or energy.
Time Period
: Duration for one complete oscillation. Inversely related to frequency.
Electromagnetic Spectrum
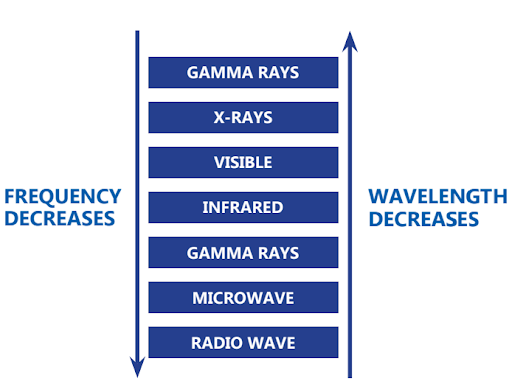
All forms of electromagnetic radiation, ranging from radio waves to gamma rays, are included in the electromagnetic spectrum. It spans a wide range of frequencies and wavelengths, including visible light. Each type of radiation has distinct properties and applications.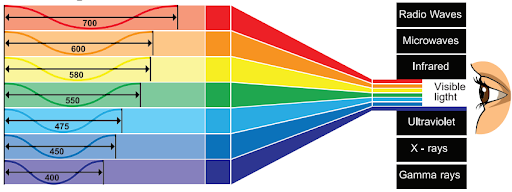
Planck’s Quantum Theory of Radiation
Planck’s Quantum Theory of Radiation is a scientific concept that explains energy behavior at the atomic and subatomic levels. It challenges the idea of continuous energy by proposing that energy exists in tiny, discrete packets called quanta. These quanta are the smallest units of energy that can be emitted or absorbed as electromagnetic radiation. According to Planck, the energy of radiation is directly proportional to its frequency, expressed as E = hν, where E represents energy, h is Planck’s constant (approximately 6.626 × 10^{-34} J·s), and ν denotes frequency.Photoelectric Effect
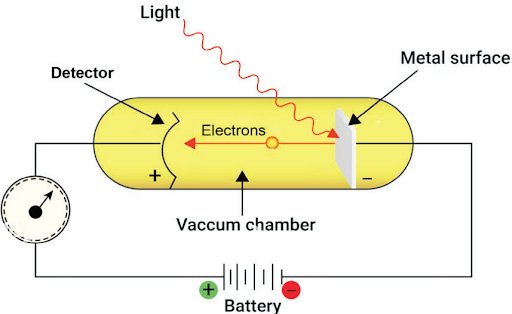
Bohr Model
Bohr’s Model of the Atom, proposed by Danish physicist Niels Bohr in 1913, revolutionized our understanding of atomic structure. Unlike earlier models, Bohr’s theory incorporated quantum principles. It depicted electrons orbiting a positively charged nucleus in fixed energy levels (shells), similar to the planets orbiting the sun. Electrons emit energy as light when they transition to lower energy levels. Although Bohr’s model successfully explained hydrogen’s spectral lines, it faced limitations, such as failing to account for magnetic and electric field effects and violating the Heisenberg Uncertainty PrincipleRadius of Bohr's Orbit (n is the principal quantum number)
r n = n^2 * h^2 * ε₀ / (π * mₑ * e²)
Where:
The nth orbit is radius is denoted by rn. h is the Planck constant, ε₀ is the vacuum permittivity, mₑ is the mass of the electron, e is the elementary charge. Velocity of an Electron in an Orbit (n is the principal quantum number)v n = k * e² / (n * h)
Where: The electron's velocity in the nth orbit is represented by v n .- k is Coulomb's constant.
T n = 2π * n * ε₀ * h² / (k * e² * mₑ)
Where:
- T n is the time period of the electron in the nth orbit.
fn = 1 / Tn
Where:- f_n is the frequency of the electron in the nth orbit.
Energy of Electron in Orbit (potential and kinetic energy)
En = -k * e² / (2 * rn)
Where:- The electron's energy in the nth orbit is denoted by En.
E total = -k * Z * e² / (2 * rn)
Where:
- E total is the total energy of the electron in a hydrogen-like species.
Atomic Spectra
Study of how electromagnetic radiation interacts with atoms and atomic ions. It explains phenomena like refraction and emission of radiation by excited atoms. Atomic spectra include emission spectra and absorption spectra.
Emission Spectrum
When electrons in excited atoms or molecules move from higher energy states to lower ones, they emit radiation. The resulting spectrum of emitted radiation at specific wavelengths is called an emission spectrum.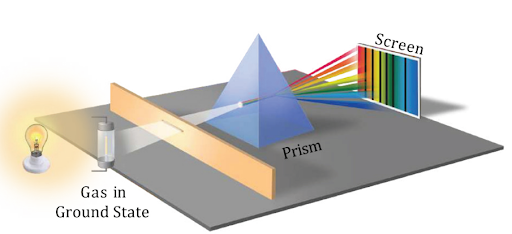
Absorption Spectrum
In an absorption spectrum, matter absorbs specific wavelengths of electromagnetic radiation. It appears as dark lines within a continuous spectrum.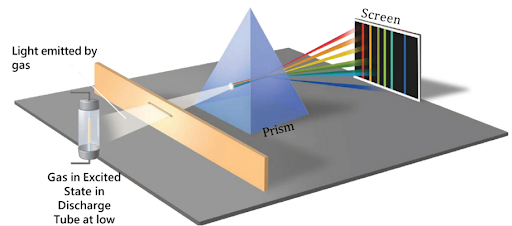
Pathway to Quantum Mechanical Model
The pathway to the quantum mechanical model of the atom evolved through seminal contributions from scientists like Schrodinger, Heisenberg, and Dirac. Integrating wave-particle duality and mathematical formulations, the model describes electrons as probability waves in specific energy levels, transforming our understanding of atomic behavior and laying the foundation for modern quantum physics.
Dual Nature of Matter
The dual nature of matter is a fundamental concept in quantum mechanics, which describes particles such as electrons exhibiting both particle-like and wave-like properties. This idea is encapsulated by the wave-particle duality principle, suggesting that particles can display characteristics of both waves and particles.HEISENBERG’S UNCERTAINTY PRINCIPLE
Heisenberg's Uncertainty Principle, formulated by Werner Heisenberg in 1927, is a key principle in quantum mechanics. It states that it is impossible to simultaneously and precisely measure the exact position and momentum (or velocity) of a particle. Mathematically, the Uncertainty Principle is expressed as:Δx * Δp ≥ ħ/2
Where: Δx is the uncertainty in position, Δp is the uncertainty in momentum, Planck constant reduction (ħ = h/(2π)) is represented by ħ. h is the Planck constant If you are looking for the best online coaching for NEET 2024, you should check out PW NEET online coaching . We offer you the most comprehensive and updated study material, expert faculty, live classes, doubt sessions, mock tests, and personalized guidance. With PW you can ace the NEET exam and achieve your dream of becoming a doctor.Structure of Atom FAQs
What is atom class 11?
What are the 7 atomic models?
What are the 4 types of atomic structure?
What is atom structure 9th?