
Actetamide formula is also known as Acetic acid amide, Ethanamide or Acetimidic acid. It is derived from acetic acid and is widely used as a plasticizer.
A hygroscopic solid with a mousy odor, ethanamide, is obtained as a hygroscopic solid. The compound is readily soluble in water, chloroform, hot benzene, glycerol, and ether but is only slightly soluble in ether. The compound is formed when acetic acid (CH3COOH) is condensed with ammonia (NH3). It is naturally found in red beetroot.Structure of Acetamide -CH3CONH2
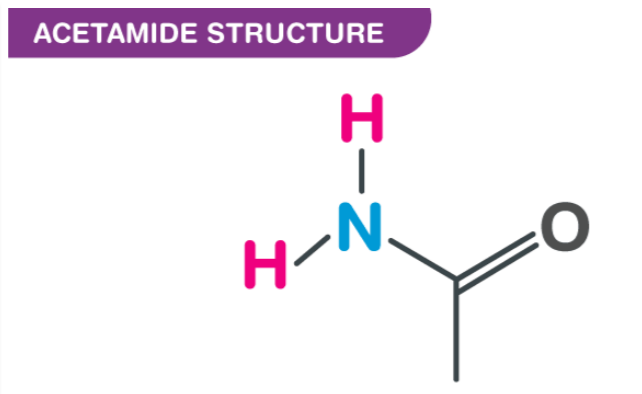 The acetamide molecule has a molar mass of 56.07g/mol and has a chemical formula C2H5NO or CH3CONH2. Acetamide has a methyl group attached to two carbonyl groups (CO) and two amine groups (NH2). In addition to having the typical carboxylic acid amide functional group of RC(=O)NH2, acetamide is a member of the primary carboxylic acid amide family. As a natural chemical, it is also present in nature.
The acetamide molecule has a molar mass of 56.07g/mol and has a chemical formula C2H5NO or CH3CONH2. Acetamide has a methyl group attached to two carbonyl groups (CO) and two amine groups (NH2). In addition to having the typical carboxylic acid amide functional group of RC(=O)NH2, acetamide is a member of the primary carboxylic acid amide family. As a natural chemical, it is also present in nature.
Also Read - Aluminium Nitrate Formula
Properties of Acetamide –CH3CONH2
| C2H5NO | Acetamide |
| Molecular weight/molar mass of C2H5NO | 59.068 g/mol |
| Density of Acetamide | 1.159 g/cm3 |
| Boiling Point of Acetamide | 221.2 °C |
| Melting Point of Acetamide | 79 to 81 °C |
Production of Acetamide-CH3CONH2
It can be produced in chemical laboratories by dehydrating ammonium acetate. [NH4][CH3CO2] → CH3C(O)NH2 + H2O The compound can also be obtained by ammonolysis of acetylacetone under the same conditions as reductive amination. In addition, anhydrous acetic acid (CH3COOH), dried hydrogen chloride gas, and acetonitrile can be prepared in an ice bath along with acetyl chloride as a reagent. Dehydrating ammonium acetate or hydrolyzing acetonitrile can be used to produce it on an industrial scale. CH3CN + H2O → CH3C(O)NH2Also Check - Bond Order Formula
Uses of Acetamide-CH3CONH2
- Many inorganic and organic compounds are soluble in acetamide.
- Explosives use this compound.
- Used as Plasticizer.
- A hygroscopic agent.
- Methylamine is manufactured with this chemical.
- Used as a Stabilizer.
- Used as a penetrating agent.
- Used as a Fire suppressant.
Also Check - Molar Volume Formula
Health Hazards of Acetamide- CH3CONH2
- Acetic acid amide is combustible and releases toxic fumes when heated.
- It can irritate mucous membranes, skin, and eyes. It can also damage corneas.
Acetamide Formula FAQs
What is acetamide, and where is it commonly found?
Acetamide is an organic compound with the chemical formula CH3CONH2. It is primarily used in the chemical industry as a precursor for various chemicals and pharmaceuticals.
Is acetamide a natural compound found in organisms?
Acetamide is not commonly found in nature and is typically synthesized through chemical processes. However, some microorganisms can produce it as a metabolic byproduct under specific conditions.
Is acetamide safe for human consumption or use in personal care products?
Acetamide is not approved for use in food products or personal care items. It is primarily an industrial chemical and should not be ingested or applied to the skin without proper safety precautions.
What are the potential health risks associated with acetamide exposure?
Exposure to acetamide may irritate the eyes, skin, and respiratory tract. Prolonged or high-level exposure can be harmful, and it is essential to follow safety guidelines and use protective equipment when handling this chemical.
What are the industrial applications of acetamide?
Acetamide serves as a building block in synthesizing various chemicals and pharmaceuticals, including pesticides, herbicides, and pharmaceutical drugs. It is used as a starting material for producing more complex organic compounds in the chemical industry.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









