
The Molar Volume Formula is crucial for mathematical calculations related to gases. One mole of a chemical element or chemical compound occupies one molar volume (Vm) at standard temperature and pressure (STP). The molar mass (M) and mass density may be divided to calculate it.
At a particular temperature and pressure, the volume of a mole of any gas is fixed. Cubic meters per mole (m3/mol) is the SI unit for molar volume. However, different units are typically used because that is a high amount. The unit cubic centimeters per mole (cm3/mol) is used for solids and liquids. The unit cubic decimeters per mole (dm3/mol) might be used for gases.Introduction to Molar Volume
The volume of one mole of a substance at standard pressure and temperature is called the molar volume. Put another way, it is the proportion of molar mass to density. It fluctuates inversely with the density of the material but is directly proportional to its mass. The perfect gas equation yields the ideal gas's molar volume.- When a sample has two or more different components, the density of the mixture is used to calculate the estimated molar volume by multiplying the molar volumes of the different components.
- Given that the mole is one of the seven SI units of measurement for the seven fundamental physical magnitudes, understanding the volume filled by a mole of a material is essential for working with it in chemistry.
- Avogadro's law states that if an ideal gas is in the middle of the same pressure and temperature conditions, its molar volume will equal that of another ideal gas in the same conditions. This is particularly true for molecular gases, where a mole contains the value of Na, known as Avogadro's number.
Introduction to Molar Volume Formula
Gas particles can be forced to envelop one another when under high pressure because they are compressible. As a result, the system has less open space and less gas. The volume of a gas is also influenced by temperature. When a gas is heated, its molecules move more quickly, causing the gas to expand. Due to the fluctuation in gas volume induced by changes in pressure and temperature, the comparison of gas volumes must be done at a consistent temperature and pressure. STP is defined as a temperature and pressure combination of 0 degrees Celcius (273.15K) and 1 atm.Also Check - Charles Law Formula
The molar volume at STP corresponds to the volume of one mole of a gas. At STP, a mole of any gas (6.021023 typical particles) takes up 22.4 L of space. [caption id="attachment_16142" align="alignnone" width="300"]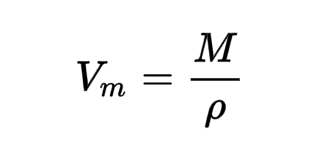 Molar volume formula[/caption]
Molar volume formula[/caption]
Download PDF Molar Volume Formula
Molar Volume Formula
The molar volume formula is written as Vm = M/ρ where, The molar volume is Vm and The molar mass of gas is M. The gas's density is ρ. The volume of a mole of a gas at STP is known to be 22.4 L. The formula is as follows when referring to the quantity of moles: Vm = 22.4 n , where the molar volume is Vm. The number of gas moles is n. For instance, take the below example for the molar volume formula. When carbon and air interact, carbon dioxide is created. O 2 (g)+C(s)=C O 2 (g) Consider the situation where we need to calculate the molar volume of carbon dioxide gas produced by the reaction of 2 g of carbon with air. It is evident from the reaction that 1 mole of carbon produces 1 mole of carbon dioxide. 2 grams of carbon are equivalent to 2/12 or 0.166 moles. Consequently, n = 0.166 mol/mol of carbon dioxide. With the result of the molar volume formula, Vm = 22.4 n = 22.4 (0.166) = 3.73 LMolar Volume Formula FAQs
What does "molar volume value" mean?
The gas's molar volume is the volume occupied by one mole of gas under standard temperature and pressure, or STP conditions (0°C and 1 atmosphere, or pressure). At STP, it has a value of 22.4 L.
What is water's molar volume?
Although one may also speak about molar quantities of substances not gaseous at STP, this volume is often intended when "molar volume" is used. The molar volume of water is approximately 18 cc because at STP, water occupies nearly 1 cc per gram, and one mole of water molecules weighs nearly 18 grams.
What does molar volume mean?
The volume filled by one mole of a chemical element or compound at standard temperature and pressure (STP) is known as the molar volume (Vm). It may be derived by dividing the mass density by the molar mass (M).
Explain the molar volume formula unit.
The molar volume uses the SI unit of cubic metres per mole (m3/mol), even though it is more typical to use cubic centimetres per mole (cm3/mol)for liquids and solids and cubic decimeters per mole (dm3/mol)for gases.
What is the STP molar volume?
The volume of one mole of a gas at STP is known as the molar volume of a gas. One mole (6.021023 typical particles) of any gas takes up 22.4L at STP.
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









