
Known as Azelaic Acid Formula, it has a saturated dicarboxylic acid found in wheat, barley, and rye. It is also known as nonanedioic acid. It can be obtained by ozonolysis of oleic acid at the industrial level, and naturally by Malassezia furfur, a yeast found on the skin. There are eight rotatable bonds in the hydrogen bond. The property values for the acceptor and donor are 2, respectively.
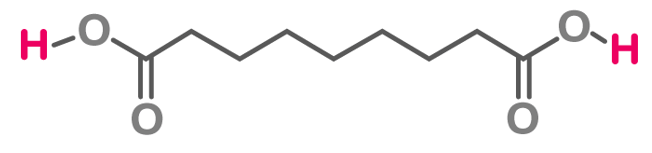
Structure of Azelaic Acid
The chemical formula of azelaic acid is C 9 H 16 O 4 , or HOOC(CH 2 ) 7 COOH, and its simplified molecular input line entry system (SMILES) is OC(=O)CCCCCCCC(O)=O. In its IUPAC name, nonanedioic acid is a dicarboxylic acid with 9 carbon atoms, 16 hydrogen atoms, and 4 oxygen atoms.
What is Azelaic Acid?
As a naturally occurring acid, Azelaic Acid boasts anti-inflammatory properties and has proven effective in addressing mild to moderate acne. By reducing the growth of bacteria and promoting exfoliation, this acid keeps pores clear. In addition to its acne-fighting capabilities, it has also been beneficial for pigmentary abnormalities, post-inflammatory hyperpigmentation, and dermatitis. Plus, its gentle exfoliation helps rid the skin of dead cells that can contribute to clogged pores. When applied topically, Azelaic Acid produces hydrogen peroxide.
Properties of Azelaic Acid
- Azelaic acid boasts various valuable properties that make it versatile in its application.
- Its features include being a white, waxy substance belonging to the dicarboxylic acids drug class with the structural formula C 9 H 13 NO 3 .
- The chemical formula is R-CH=CH-CO2H and has a molecular weight of 161.197 g/mol.
- In addition, this organic compound has a melting point of 100 °C and a boiling point of 232 °C, comprising carbon, hydrogen, and oxygen atoms. Moreover, saturated azelaic acid presents itself as a colorless solid with a butter-like scent and comes in three different crystalline forms: alpha, beta, and gamma.
- Two common naturally-occurring isomers are alpha-azelaic acid (AzA) and beta-azelaic acid (BA).
- As a bonus, this naturally occurring dicarboxylic acid possesses potent abilities against malarial parasites.
Also Check – Ionization Energy Formula
Applications of Azelaic Acid
This acid’s esters have many applications, including lubricants and plasticisers. Azelaic acid is a thickening agent in lithium complex grease in the lubricant industry. Azelaic acid reacts with hexamethylenediamine to form Nylon-6,9, a plastic with specific applications.
Dermatologists use Azelaic Acid Formula to treat mild to moderate acne and inflammatory and comedonal acne. It belongs to a medication class known as dicarboxylic acids. Furthermore, it works by killing acne bacteria that cause blockages in skin pores. Furthermore, it reduces keratin production, which is a natural substance that promotes the growth of acne bacteria.
Azelaic acid possesses antibacterial, keratolytic, comedolytic, and anti-oxidant properties, making it a versatile treatment for mild to moderate acne. This powerful ingredient eliminates the bacteria responsible for acne, reducing inflammation and improving conditions such as rosacea. It is also effective in treating skin pigmentation and lightening areas of discoloration caused by melanin. Additionally, azelaic acid plays a role in preventing microbial protein synthesis and is used as a base for various industrial products such as polymers and plasticizers. In fact, it is often utilized as a thickening agent in polymer industries and can be found in the form of esters, which serve as both plasticizers and lubricants.
Also Check – Molecular Speed Formula
Production of Azelaic Acid
Natural dicarboxylic acids such as azelaic acid can be found in wheat, rye, and barley. It can be produced naturally by Malassezia furfur, a yeast that lives on normal skin, but industrially is produced by ozonolysis of oleic acid. Industrially, ozonolysis of oleic acid produces azelaic acid and pelargonic acid (nonanoic acid). By bacterial degradation, pelargonic acid (nonanoic acid) becomes azelaic acid.
Azelaic Acid Formula FAQs
Q1. What is the chemical formula of azelaic acid?
Q2. What is azelaic acid used for in skincare?
Q3. Is azelaic acid available over the counter or by prescription?
Q4. Are there any common side effects of using azelaic acid on the skin?
Q5. Can azelaic acid be used with other skincare products?










