While we might take them for granted in our everyday activities, it is important to understand how they work at a fundamental level.
In this post, we will discuss the key concepts relating to acids, bases, and salts as outlined in Chapter 2 of the CBSE Class 10 Science Syllabus.
Acid Base and Salt Class 10 Notes
Acid Base and Salt Class 10 Notes help you in better preparation for board exams. Understanding their properties, reactions, and applications is crucial in the study of chemistry.1. Acids
Chemical Definition:
An acid is a substance that produces hydrogen ions (H⁺) as the only positive ions in an aqueous solution.
Physical Properties:
-
Taste: Sour (khatta)
-
Corrosive in nature
Indicator Behaviour:
-
Turns blue litmus paper red
Common Examples:
| Chemical Formula | Name |
|---|---|
| HCl | Hydrochloric Acid |
| H₂SO₄ | Sulphuric Acid |
| HNO₃ | Nitric Acid |
| CH₃COOH | Acetic Acid (Vinegar) |
2. Bases
Chemical Definition:
A base is a substance that increases the concentration of hydroxyl ions (OH⁻) in an aqueous solution.
Physical Properties:
-
Taste: Bitter (kadwa)
-
Feel: Soapy or slippery to touch
Indicator Behaviour:
-
Turns red litmus paper blue
Alkalis:
Bases soluble in water are called alkalis, such as NaOH and KOH.
Common Examples:
| Chemical Formula | Name |
|---|---|
| NaOH | Sodium Hydroxide |
| KOH | Potassium Hydroxide |
| NH₄OH | Ammonium Hydroxide |
| Mg(OH)₂ | Magnesium Hydroxide |
| Ca(OH)₂ | Calcium Hydroxide |
3. Indicators
Indicators are substances used to detect whether a solution is acidic or basic.
Common Indicators:
| Indicator | Colour in Acid | Colour in Base |
|---|---|---|
| Litmus | Red | Blue |
| Turmeric | Red | Yellow |
| Phenolphthalein | Colourless | Pink |
| Methyl Orange | Red | Yellow |
Olfactory Indicators
These are substances whose smell changes depending on the medium. They are particularly useful for visually impaired students.
-
Retain smell in acidic solution
-
Lose smell in the basic solution
Examples: Onion, Vanilla essence, Clove oil
Acids and Bases in Water
Hydronium Formation:
-
Acids produce H⁺ ions in water, which combine with water molecules to form hydronium ions (H₃O⁺).
-
Example: HCl + H₂O → H₃O⁺ + Cl⁻
Bases in Water:
-
Bases increase OH⁻ ions in water. Water-soluble bases like NaOH and KOH are called alkalis.
Dilution of Acid:
-
Adding concentrated acid to water is highly exothermic.
-
Always add acid to water slowly with stirring to prevent splashing.
Electrical Conductivity:
-
Aqueous solutions of acids and bases conduct electricity due to free ions.
-
Substances like glucose and alcohol do not ionize and hence do not conduct electricity.
Important Reactions
a) Reaction with Metals:
-
Acids react with active metals to produce salt and hydrogen gas.
-
Example: Zn + H₂SO₄ → ZnSO₄ + H₂↑
-
-
Some bases (like NaOH) also react with metals:
-
2NaOH + Zn → Na₂ZnO₂ + H₂↑
-
b) Reaction with Carbonates and Hydrogen Carbonates:
-
Acid + Metal Carbonate → Salt + H₂O + CO₂
-
Acid + Metal Hydrogen Carbonate → Salt + H₂O + CO₂
-
Test: CO₂ turns limewater milky due to CaCO₃ formation.
c) Neutralization Reaction:
-
Acid + Base → Salt + Water
-
Example: HCl + NaOH → NaCl + H₂O
d) Reactions of Oxides:
-
Metal oxides are generally basic and react with acids to form salt and water.
-
Non-metal oxides are generally acidic and react with bases to form salt and water.
Strength of Acids and Bases
-
Strong acids (e.g., HCl, H₂SO₄) release high concentration of H⁺ ions.
-
Weak acids (e.g., CH₃COOH) release low concentration of H⁺ ions.
-
Strong bases (e.g., NaOH, KOH) release high OH⁻ ions.
-
Weak bases (e.g., NH₄OH) release low OH⁻ ions.
pH Scale
- Definition: The pH scale measures the acidity or basicity of a solution.
- pH Values:
- 0 to 7: Acidic (lower values are more acidic)
- 7: Neutral
- 7 to 14: Basic or alkaline (higher values are more basic)
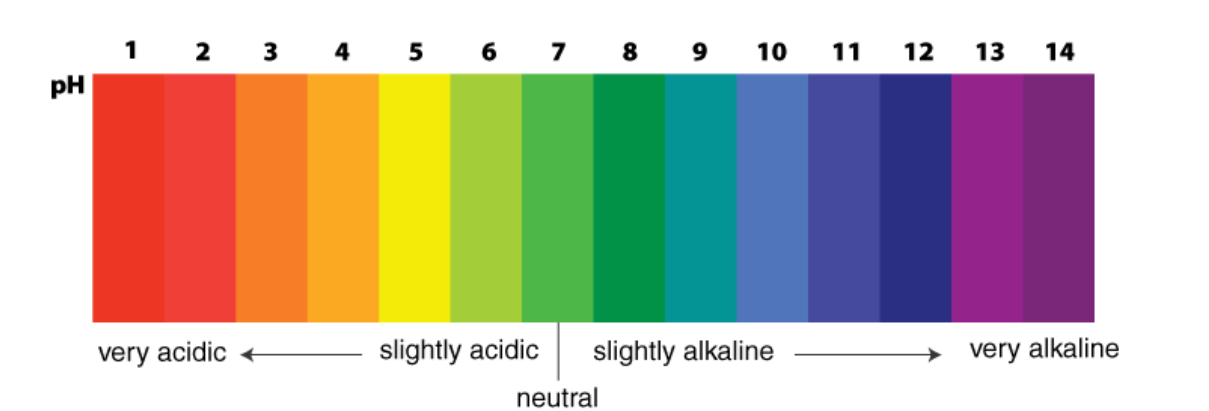
Importance of pH in Everyday Life
The pH scale measures the concentration of hydrogen ions (H⁺) in a solution and ranges from 0 to 14. It is an important parameter in biological, environmental, and industrial processes. Maintaining the proper pH is crucial for human health, the environment, and daily activities.
1. Human Body
-
The normal pH range of human blood is 7.0 to 7.8, which is slightly basic.
-
Proper pH is essential for enzyme activity and overall metabolism.
-
Digestive acids: The stomach produces hydrochloric acid (HCl) to help digest food.
-
Excess stomach acid can cause indigestion, irritation, and pain.
-
To neutralize excess acid, antacids like milk of magnesia (Mg(OH)₂) or baking soda are used.
2. Acid Rain and Environment
-
Rainwater normally has a pH of about 5.6.
-
If the pH drops below 5.6 due to pollutants like SO₂ and NO₂, it becomes acid rain.
-
Acid rain affects rivers and lakes, making it difficult for aquatic life to survive.
-
It can also damage crops, soil quality, and buildings.
3. Dental Health
-
When the pH in the mouth falls below 5.5, it can lead to tooth decay.
-
Toothpaste, which is slightly basic, helps neutralize acids and protect teeth.
4 Honey Bee Sting:
-
The sting contains acidic compounds like formic acid or methanoic acid.
-
Treatment: Apply a mild base, such as baking soda (NaHCO₃), to neutralize the acid and relieve pain.
5. Nettle Stings:
-
The stinging hairs of nettle release methanoic acid, causing pain on contact.
-
Natural Remedy: Rub the affected area with dock plant leaves, which naturally neutralize the acid and reduce irritation
Indicators:
- Definition: Indicators are substances that change color in the presence of acids or bases.
- Examples of Indicators:
- Litmus paper (blue turns red in acid, red turns blue in base)
- Phenolphthalein (colorless in acid, pink in base)
- Methyl orange (red in acid, yellow in base)
Neutralization:
- Definition: Neutralization is the reaction between an acid and a base, resulting in the formation of salt and water.
- General Equation: Acid + Base → Salt + Water
Applications:
- Acids:
- Used in industries, laboratories, and daily life (e.g., citric acid in fruits).
- Bases:
- Used in the manufacture of soaps and detergents.
- Salts:
- Have various applications, including in food preservation and water treatment.
Key Industrial Compounds and Processes
Chlor-Alkali Process
The Chlor-Alkali Process is an important industrial method for producing sodium hydroxide (NaOH), chlorine gas (Cl₂), and hydrogen gas (H₂).
-
Process: Electrolysis of brine (concentrated NaCl solution) decomposes sodium chloride into NaOH, Cl₂, and H₂.
-
Importance: NaOH is widely used in soap, paper, and chemical industries; Cl₂ is used in disinfectants and bleaching; H₂ is used as a fuel and in ammonia production.
Bleaching Powder (CaOCl₂)
Preparation: Produced by reacting calcium hydroxide [Ca(OH)₂] with chlorine gas (Cl₂):
Ca(OH)₂ + Cl₂ → CaOCl₂ + H₂O
Uses:
-
Disinfecting drinking water and swimming pools
-
Bleaching cotton, linen, and paper
-
Acting as a strong oxidising agent in chemical industries
Baking Soda (NaHCO₃)
Preparation: Baking soda is prepared using sodium chloride (NaCl), ammonia (NH₃), carbon dioxide (CO₂), and water (H₂O) in the Solvay process.
Uses:
-
As an antacid to neutralize excess stomach acid
-
In baking, it helps dough rise
-
Used in fire extinguishers as it releases CO₂ gas when heated
Baking Powder
Composition: A mixture of baking soda (NaHCO₃) and a mild edible acid such as tartaric acid.
Action:
-
On mixing with water, it releases carbon dioxide (CO₂) gas, which makes cakes and bread soft and fluffy.
Washing Soda (Na₂CO₃·10H₂O)
Preparation: Obtained by heating sodium carbonate (from soda ash) and then recrystallizing to obtain the decahydrate form.
Uses:
-
Cleaning agent for removing grease and stains
-
Softens hard water, making it suitable for domestic and industrial purposes
Water of Crystallization
-
Some salts contain a fixed number of water molecules in their crystal structure.
-
Example: Gypsum (CaSO₄·2H₂O) contains 2 water molecules per formula unit.
-
This water is essential for maintaining the crystal structure of the salt.
Plaster of Paris (POP)
Preparation: Heating gypsum (CaSO₄·2H₂O) at 373 K partially removes water, producing CaSO₄·½H₂O.
Uses:
-
Making toys, decorative items, and sculptures
-
Smoothing surfaces in interior decoration
-
Medical use: casts for fractures
Acid Base and Salt Notes PDF
Understanding the concepts of acids, bases, and salts plays a crucial role in mastering the subject of CBSE Class 10 Science. From learning about their properties, reactions, and uses to exploring real-life examples and solving practice questions, this chapter offers a diverse range of information for students to grasp.By providing a free PDF download of acids bases and salts class 10 notes, we hope to make the learning process easier and more accessible for all students.
Acid Base and Salt Class 10 Notes PDF
Related Links
