what is pH Scale
Acid Base And Salt of Class 10
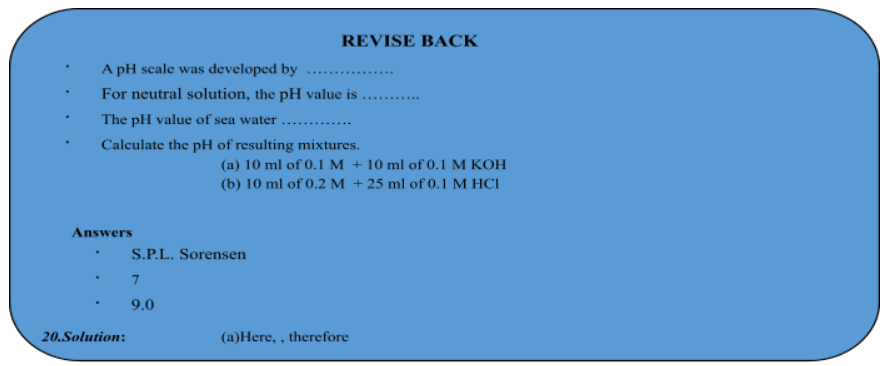
pH SCALE
Acidic strength means the tendency of an acid to give H + ions in water and basic strength means the tendency of a base to give OH − ions in water. So more the tendency to give H + or OH − ions, more will be the acidic or basic strength of acid or base.
Many properties of aqueous solutions depend on the concentration of H + ions of the solutions and therefore there is a need to express these concentrations in simpler terms. For this purpose, Sorenson introduced the concept of pH.
pH = – log aH + (where aH + is the activity of H + ions).
Activity of H + ions is the concentration of free H + ions in a solution. By free, we mean those that are at a large distance from the other ion so as not to experience its pull. We can infer from this that in dilute solutions, the activity of an ion is same as its concentration since more number of solvent molecules would separate the two ions. For concentrated solutions, the activity would be much less than the concentration itself.
Therefore, the earlier given expression of pH can be modified for dilute solutions as, pH = – log [H + ]. This assumption can only be made when the solution is very much dilute, i.e. pH scale: A scale developing for measuring hydrogen ion concentration in a solution called pH scale, has been developed by S.P.L. Sorensen. The P in pH stands for 'potenz’ in German power. On the pH scale we can measure pH from O (very acidic) to 14(very alkaline). pH should be thought of simply as a number which indicates the acidic or basic nature of solution. Higher the hydrogen ion concentration, Lower is the pH scale.
Characteristic of pH scale are –
- For acidic solution, pH < 7
- For alkaline solution, pH > 7
- For neutral solution, pH = 7
p[H] = − log [H + ]
P[OH] = − log [OH − ]
When [H + ] of a solution is more than [OH − ], the solution is said to be acidic. When [H + ] of a solution is less than [OH − ], the solution is said to be basic. If [H + ] and [OH − ] of a solution are equal, the solution is called neutral.
Self Ionisation of Water
Pure water acts as a very weak electrolyte i.e. it gets ionised very weakly to give H
+
and OH
−
ions, i.e. H2O
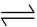 H
+
+ OH
−
. Now, applying law of chemical equilibrium.
H
+
+ OH
−
. Now, applying law of chemical equilibrium.
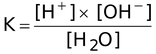 where K is equilibrium constant called dissociation constant of water
where K is equilibrium constant called dissociation constant of water
K × [H 2 O] = [H + ] × [OH − ]
Kw = [H + ] × [OH − ] where Kw is ionic product of water.
−log Kw = −log [H + ] − log [OH − ]
pKw = pH + pOH
Since the concentration of pure water is constant, so when it is multiplied by K product will also be a constant called ionic product of water (Kw) which is constant at constant temperature. The value of Kw is approximately 10 -14 at 298 K.
Therefore, [H + ] × [OH − ] = 10 -14 at 298 K
Based on Kw value of water at 298 K, S.P.L. Sorenson introduced a scale called pH scale or pOH scale to measure the acidic or basic strength of acids or bases. The range of the pH scale s from 0 to 14.
As ionization of water gives equal amount of H + and OH − , we can say
[H + ] × [H + ] = 10 -14 or [OH − ] × [OH − ] = 10 -14
[H + ] = 10 -7 or [OH − ] = 10 -7
pH = -log10 -7 or pOH = - log 10 -7
pH = 7 or pOH = 7
For pure water, at all temperatures
[H + ] 2 = [OH - ] 2 = Kw
[H + ] = [OH − ] = √K w
pH = pOH = - log √K w
For pure water [H + ] is always equal to [OH − ] so it is called neutral. So, neutral pH of any solution at 298 K taking water as solvent is 7. For acidic or basic solutions the [H + ] and [OH − ] are not equal but their product is always equal to Kw at that temperature. The variation of Kw with temperature is expressed as
log
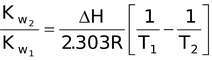
where K w2 and K w1 are the ionic product of water at temperature T2 and T1 respectively, ΔH is the enthalpy change for ionization of H2O into H + and OH − ions and R is the molar gas constant.
Common ion effect
If an ionic equilibrium, we add any ion which appears in the equilibrium reaction, the equilibrium will shift in a direction opposite to that in which that ion appears.
So dissociation of weak acids and bases is suppressed in presence of strong acids and bases respectively. Similarly the solubility of salts decreases in presence of any common ion of that salt.
Determination of pH of Acids
Strong Acid
A strong acid is that which ionises completely to give maximum H + ions which it is capable of giving. e.g.
HCl → H + + Cl −
Let the concentration of HCl be c. So the [H + ] coming from acid is also c.
Therefore pH = −log c
Now let us calculate the pH of 10 -3 M HCl, 10 -4 M HCl, 10 -5 M HCl, 10 -6 M HCl and 10 -7 M HCl.
For 10 -3 MHCl,
HCl → H + + Cl −
10 -3 M
pH = −log 10 -3 = 3
For 10 -4 MHCl
pH = −log10 -4 = 4
For 10 -5 MHCl
pH = −log 10 -5 = 5
For 10 -6 MHCl
pH = −log 10 -6 = 6
Universal indicator papers for pH values:
Indicators like litmus, phenolphthalein and methyl orange are used in predicting the acidic and basic characters of the solutions. However universal indicator papers have been developed to predict the pH of different solutions. Such papers represent specific colours for different concentrations in terms of pH values.
The exact pH of the solution can be measured with the help of pH meter which gives instant reading and it can be relied upon.
pH values of a few common solutions are given below :
|
Solution |
Approximate pH |
Solution |
Approximate pH |
|
Gastric Juices |
1.0 - 3.0 |
Pure water |
7.0 |
|
Lemon juices |
2.2-2.4 |
Blood |
7.36-7.42 |
|
Vinegar |
3.0 |
Baking soda solution |
8.4 |
|
Beer |
4.0-5.0 |
Sea water |
9.0 |
|
Tomato juice |
4.1 |
Washing soda solution |
10.5 |
|
Coffee |
4.5-5.5 |
Lime water |
12.0 |
|
Acid rain |
5.6 |
House hold ammonia |
11.9 |
|
Milk |
6.5 |
Sodium hydroxide |
14.0 |
|
Saliva |
6.5-7.5 |
Significance of pH in daily life:
(i) pH in our digestive system: Dilute hydrochloric acid produced in our stomach helps in the digestion of food. However, excess of acid causes indigestion and leads to pain as well as irritation. The pH of the digestive system in the stomach will decrease. The excessive acid can be neutralised with the help of antacid which are recommended by the doctors. Actually, these are group of compounds (basic in nature) and have hardly any side effects. A very popular antacid is 'Milk of Magnesia' which is insoluble magnesium hydroxide. Aluminium hydroxide and sodium hydrogen carbonate can also be used for the same purpose. These antacids will bring the pH of the system back to its normal value. The pH of human blood varies from 7.36 to 7.42. It is maintained by the soluble bicarbonates and carbonic acid present in the blood. These are known as buffers.
(ii) pH change leads to tooth decay: The white enamel coating on our teeth is of insoluble calcium phosphate which is quite hard. It is not affected by water. However, when the pH in the mouth falls below 5.5 the enamel gets corroded. Water will have a direct access to the roots and decay of teeth will occur. The bacteria present in the mouth break down the sugar that we eat in one form or the other to acids; Lactic acid is one of these. The formation of these acids causes decrease in pH. It is therefore advisable to avoid eating sugary foods and also to keep the mouth clean so that sugar and food particles may not be present. The tooth pastes contain in them some basic ingredients and they help in neutralising the effect of the acids and also increasing the pH in the mouth.
(iii) Role of pH in curing stings by insects: The stings of bees and ants contain methanoic acid (or formic acid). When stung, they cause lot of pain and irritation. The cure is in rubbing the affected area with soap. Sodium hydroxide present in the soap neutralises acid injected in the body and thus brings the pH back to its original level bringing relief to the person who has been stung. Similarly, the effect of stings by wasps containing alkali is neutralised by the application of vinegar which is ethanoic acid (or acetic acid)
(iv) Soil pH and plant growth: The growth of plants in a particular soil is also related to its pH. Actually, different plants prefer different pH range for their growth. It is therefore, quite important to provide the soil with proper pH for their healthy growth. Soils with high iron minerals or with vegetation tend to become acidic. The soil pH can reach as low as 4.The acidic effect can be neutralised by 'liming the soil' which is carried by adding calcium hydroxide. These are all basic in nature and have neutralising effect. Similarly, the soil with excess of lime stone or chalk is usually alkaline. Sometimes, its pH reaches as high as 8.3 and is quite harmful for the plant growth. In order to reduce the alkaline effect, it is better to add some decaying organic matter (compost or manure). The soil pH is also affected by the acid rain and the use of fertilizers. Therefore soil treatment is quite essential.