
Gram Atomic And Gram Molecular Mass
Atom and Molecule of Class 9
Gram Atomic Mass
The amount of a substance in grams which is numerically equal to the atomic mass of that substance, is known as gram atomic mass of that substance. If we want to write the gram atomic mass of a substance, we write its atomic mass, remove the atomic mass unit u, and add grams to the numerical value of the atomic mass.
Number of gram atoms
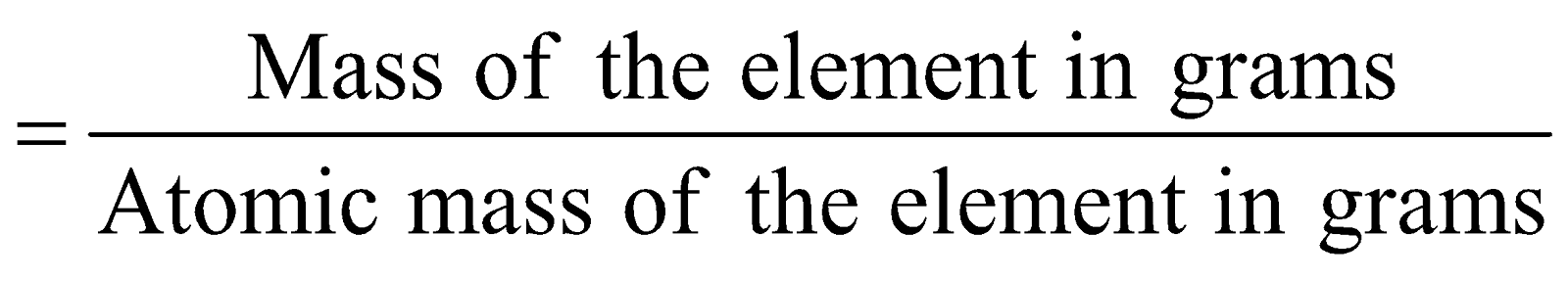
e.g. Atomic mass of nitrogen, (N) = 14 u
So, gram atomic mass of nitrogen = 14 grams
Molar Mass :
The molar mass of a substance is the mass of 1 mole, i.e. 6.023 × 10 23 particles, of that substance. Its unit is gram per mole, i.e. g/mol. The molar mass of an element is its atomic mass expressed in g/mol and the molar mass of a compound is its molecular mass expressed in g/mol.
e.g. The atomic mass of sodium (Na) is 23 u, so the molar mass of the element sodium (Na) is 23 g/mol.
Gram Molecular Mass:
The amount of a substance in grams which is numerically equal to the molecular mass of that substance, is known as gram molecular mass of that substance. If we want to write the gram molecular mass of a substance, we write its molecular mass, remove the molecular mass unit u, and add grams to the numerical value of the molecular mass.
e.g. gram molecular mass of oxygen gas (O 2 ) is 32 g.
Number of gram molecules =

Gram molecular mass should not be confused with the mass of one molecule of the substance in grams. The mass of one molecule of a substance is known as its actual mass or molecular mass.
MOLE CONCEPT:
In our daily life we buy some products in terms of numbers such as dozens (for 12), or gross (for 144) or in terms of mass such as kilograms (1000g) or quintals (100kg).
e.g. We buy bananas, oranges, mangoes etc. in dozens where as rice, wheat, pulses etc. in kilograms.
1 dozen banana = 12 banana
1 dozen mango = 12 mangoes
1 kilogram wheat = 1000g wheat
1 quintal rice = 100 kilogram rice and so on.
We cannot buy rice, pulses, sugar etc. in terms of numbers as they are very small in size. Similarly atoms and molecules are also very small in size. They are so small that we cannot see them through our eyes.
So, to express a definite amount of a chemical substance a new bigger unit “mole” was introduced. In Latin ‘mole’ means ‘heap’ or ‘collection’ or ‘pile’. The mole may be expressed in terms of mass or in terms of number.
Moles of Atoms:
- 1 mole atoms of any element occupy a mass which is equal to the gram atomic mass of that element.
- The symbol of an element represents 6.023 × 10 23 atoms (1 mole of atoms) of that element.
e.g. Symbol N represents 1 mole of nitrogen atoms and 2N represents 2 moles of nitrogen atoms.
The terms mole was introduced by Ostwald in 1896.
Moles of Molecules :
(i) 1 mole molecules of any substance occupy a mass which is equal to the gram molecular mass of that substance.
e.g. 1 mole of water (H 2 O) molecules weight equal to the gram molecular mass of water (H 2 O), i.e. 18 grams.
(ii) The formula of compound represents 6.023 × 10 23 molecules (1 mole of molecules) of that compound.
e.g. Symbol H 2 O represents 1 more of water molecules and 2H 2 O represents 2 moles of water molecules.
|
|
The symbol H 2 O does not represent 1 mole of H2 molecules and 1 mole of O atoms. Instead, it represents 2 moles of hydrogen atoms and 1 mole of oxygen atoms. |
The SI unit of the amount of a substance is Mole.
Mole in Terms of Volume :
Volume occupied by 1 gram molecular mass or 1 mole of a gas under standard conditions of temperature and pressure (273 K and 1 atm) is called gram molecular volume. Its value is 22.4 litres for each gas.
1 Mole = 1 Gram molecular mass
= 22.4 litre (at NTP)
= 6.023 × 10 23 molecules










