
Classification Of Organic Compounds
Carbon And Its Compound of Class 10
CLASSIFICATION OF ORGANIC COMPOUNDS
Classification of Organic compounds
All the known organic compounds have been divided into the following classes.
(I) Acyclic compounds
Organic compounds in which all the carbon atoms are linked to one another to form open chains (straight or branched) are called acyclic or open chain compounds. These may be either saturated or unsaturated. For example,
CH 3 CH 2 CH 2 CH3 (CH 3 ) 2 CHCH 3 CH 3 CH 2 CH = CH 2 (CH 3 ) 3 C − C ≡ CH
Butane Isobutane 1−Butene 3,3−Dimethyl−1−butyne
(II) Cyclic compounds
Cyclic compounds contain at least one ring or closed chain of atoms. These are of two types:
(i) Homocyclic compounds contain rings which are made up of only one kind of atoms. If all the atoms in the ring are carbon atoms, they are called carbocyclic compounds. These are of two types
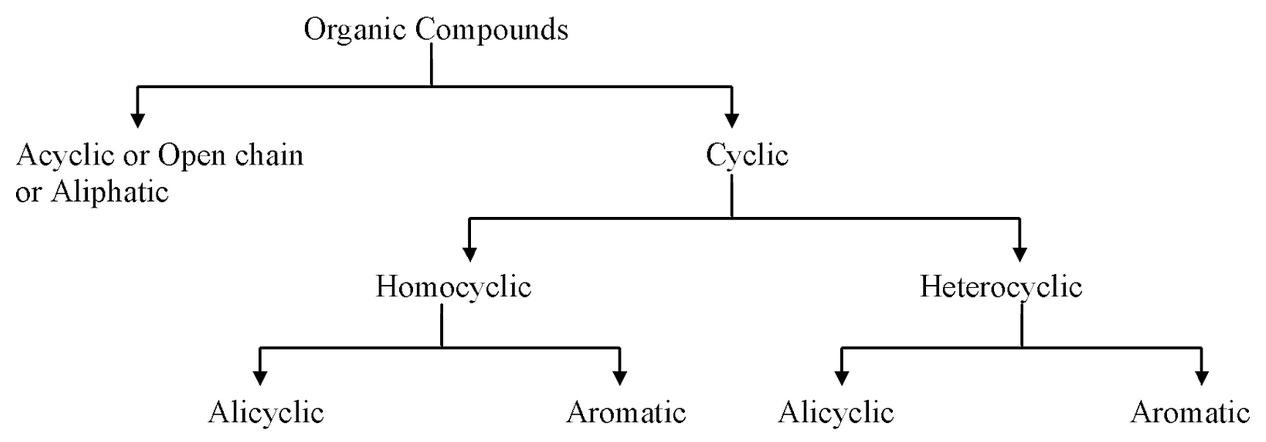
(a) Alicyclic compounds are carbocyclic compounds which resemble aliphatic compounds in their properties. For example,
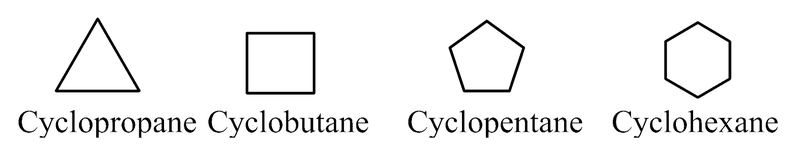
(b) Organic compounds containing one or more fused or isolated benzene rings are called aromatic compounds, For example,
These are also called benzenoid compounds or arenas.
(ii) Heterocyclic compounds: Cyclic compounds containing one or more heteroatoms (e.g. O, N, S etc.) in the ring are called heterocyclic compounds. These are of two types.
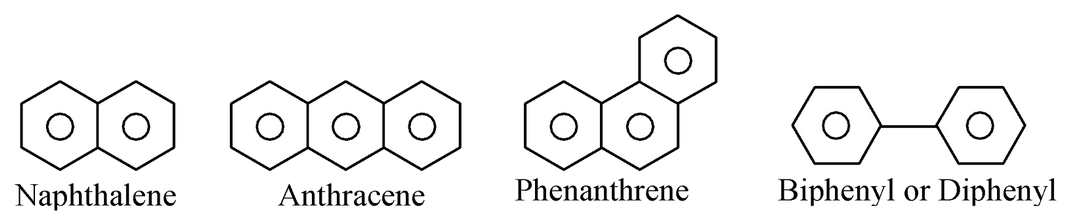
(a) Alicyclic heterocyclic compounds : Heterocyclic compounds which resemble aliphatic compounds in their properties are called alicyclic heterocyclic compounds. For example,
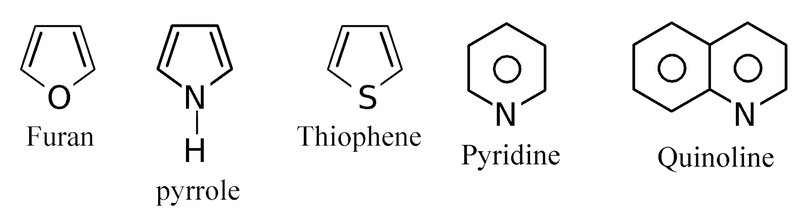
(b) Aromatic heterocyclic compounds: Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called aromatic heterocyclic compounds. For example.
Functional group
An atom or a group of atoms present in a molecule which determines its chemical properties. For example, −OH (hydroxy), −CHO (aldehydic), −COOH (carboxylic) etc.
HOMOLOGOUS SERIES
All compounds having different molecular formula but having the same functional group in their molecules possess similar properties. For example, compounds containing (−COOH) group are called acids.
HCOOH Formic acid
CH 3 COOH Acetic acid
CH 3 CH 2 COOH Propionic acid
Such structurally related compounds having similar properties are said to form a 'class'. A series of compounds of each class when arranged in order of their molecular weights are said to constitute a series called homologous series and the individual members are called 'homologous' (Greek: homus = same, logas = ratio). The property by virtue of which a number of organic compounds form a homologous series is termed 'homology'
On examination of the various homologous series we find that the members of each series
- are represented by a general formula e.g. CnH 2 n +2 represents the paraffin (alkane) series where n = 1, 2, 3, 4, …..
- form a series in arithmetical progression with a common difference of −CH 2 in their molecular formula any two consecutive members of a series differ in molecular formula by − CH2.
- arecharacterised by the presence of a characteristic similarly in their chemical properties.
- possess physical properties which vary gradually and more or less regularly in a particular order with respect to increase in molecular weight.
- can be prepared by some general reactions called general methods of preparations.
We can say that the study of organic chemistry is mainly the study of homologous series. Once the general properties and mode of preparation of a series are known it is possible to predict fairly accurately the preparation and properties of any member.
The general formulae of some homolgous series and their functional groups is given in the table.
Organic Class of Compounds |
General Molecular Formula |
|
Alkane |
CnH 2n + 2 |
|
Alkene |
CnH 2n |
|
Alkyne |
CnH 2n − 2 |
|
Diene |
CnH 2n − 2 |
|
Alkyl halide |
CnH 2n + 1X |
|
Alcohol |
CnH 2n + 2O |
|
Ether |
CnH 2n + 2 O |
|
Aldehyde |
CnH 2n O |
|
Ketone |
CnH 2n O |
|
Carboxylic acids |
CnH 2n O 2 |
|
Esters |
CnH 2n O 2 |
|
Carbonyl Chloride |
CnH 2n − 1OCl |
|
Anhydride |
CnH 2n − 2O 3 |
|
Amide |
CnH 2n + 1ON |
|
Amine |
CnH 2n + 3N |
|
Nitrile |
CnH 2n − 1N |
|
Isonitrile |
CnH 2n − 1N |
|
Nitro |
CnH 2n + 1NO 2 |
|
Nitrite |
CnH 2n + 1ONO |









