
General Methods Of Preparation
Carboxylic Acid of Class 12
1. Oxidation of alcohols, aldehydes and ketones
Alcohol is a compound consisting of one, two, or more hydroxyl (-OH) compounds attached to a single bond alkane. These compounds have a standard formula - OH. They have great importance in organic chemistry as they can be modified or adapted into different types and compounds such as Aldehydes and Ketones, etc. The reaction to alcohol is two different stages. This reaction may leave an R-O bond or may leave an O-H bond.
The process of oxidation convert alcohols into aldehydes and ketones. This reaction is one of the most important reactions in the field of organic chemistry.

Adding alcohols to aldehyde and ketones is one of the main reactions in synthetic organic chemistry. This reaction occurs when there are catalysts and the best oxidants required for this conversion, with high valent ruthenium acting as a catalyst for this type of reaction.
2. Oxidation of alkyl benzenes
Although benzene and alkane are quite unreative towards the usual oxidizing agents (KMnO 4 , K 2 Cr 2 O7 etc). The benzene ring renders an aliphatic side chain quite susceptible to oxidation. The side chain is oxidised down to the ring and only a carboxyl group (⎯COOH) remains to indicate the position of the original side chain. Potassium permanganate is generally used for this purpose, although potassium dichromate or dilute nitric acid can also be used. (Oxidation of a side chain is more difficult, however, than oxidation of an alkene and requires prolonged treatment with hot KMnO 4 )
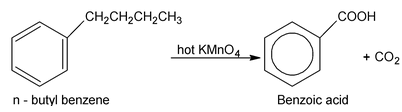
This reaction is used for two purposes (a) synthesis of carboxylic acids and (b) identification of alkyl benzenes.
3. Carbonation of Grignard reagents
The Grignard synthesis of a carboxylic acid is carried out by bubbling gaseous CO 2 into the ether solution of the Grignard reagent or by pouring the Grignard reagent on crushed dry ice (solid CO2). In the latter method dry ice serves not only as reagent but also as cooling agent.
The Grignard reagent adds to the carbon – oxygen double bond of CO2 just as in the reaction with aldehydes and keotnes. The product is the magnesium salt of the carboxylic acid, from which the free acid is liberated by treatment with mineral acid.
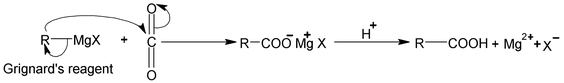
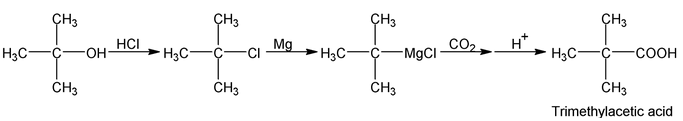
The Grignard’s reagent can be prepared from primary, secondary, tertiary or aromatic halides. The method is limited only by the presence of other reactive group in the molecule. The following synthesis illustrate the application of this method.
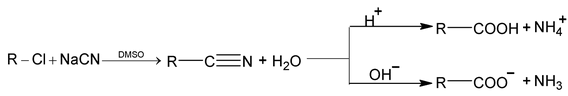
4. Hydrolysis of nitriles
Aliphatic nitriles are prepared by treatment of alkyl halides with sodium cyanide in a solvent that will dissolve both reactants. In dimethyl sulfoxide (DMSO), reaction occurs rapidly and exothermically at room temperature. The resulting nitrile is then hydrolysed to the acid by boiling with aqueous alkali or acid.
5. Use of alkoxide
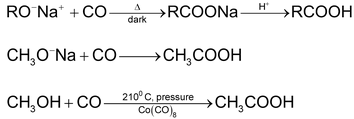
6. Carbonylation of alkenes
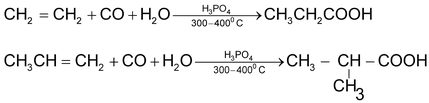
7. Oxidative cleavage of alkenes, alkynes and cyclo alkenes
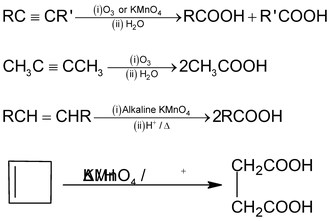
Frequently Asked Question (FAQs)
Q1. What is oxidation of ethanol?
Ans. Alcohol oxidation is oxidation about hydrogen conversion. Alcohol is oxidized due to hydrogen depletion. In hydrocarbon chemistry, oxidation and reduction of hydrogen transfer are common. Ethanol is oxidized to form ethanal aldehyde sodium dichromate (Na 2 Cr 2 O 7 ) with dilute sulfuric acid.
Q2. Why are tertiary alcohols not oxidised?
Ans. Potassium dichromate(VI) or acidified sodium solution does not oxidize tertiary alcohol. No reaction occurred. No hydrogen atom is bound to carbon in high alcohol. To form a double carbon-oxygen bond, you need to eliminate those two different hydrogen atoms.
Q3. What do secondary alcohols oxidised to?
Ans. An important oxidation reaction to organic chemistry is the second concentration of alcohol in ketones. It is converted to ketone as the second alcohol is oxidized. As well as the hydrogen bond to the second carbon, hydrogen from the hydroxyl group is lost.
Q4. Can alcohols be oxidized?
Ans. In organic chemistry, the oxidation of alcohol is an important mechanism of the reaction. To form aldehydes and carboxylic acids, primary alcohol can be oxidized; secondary alcohol can be oxidized to bring ketones. High alcohol, on the other hand, cannot be oxidized without breaking the C-C binding molecules.
Also Check




