Oxidation And Reduction
Chemical Reaction And Equation of Class 10
There are several definitions of oxidation and reduction, of which, some are
- Oxidation is the addition of oxygen, whereas, reduction is removal of oxygen.
- Oxidation is removal of hydrogen, whereas, reduction is addition of hydrogen.
- Oxidation is loss of electron, whereas, reduction is gain of electron.
- Oxidation is increase in oxidation state, whereas, reduction is decrease in oxidation state.
REDOX REACTION
Oxidation and reduction are complementary to each other and one cannot take place in the absence of the other. So the oxidation and the reduction will take place simultaneously. It is obvious that, if a substance takes electrons, there must be another substance to give these electrons. The reactions, which involve oxidation and reduction are called
redox reactions
. The redox reactions can be split into two half reactions namely oxidation half reaction (where oxidation takes place) and reduction half reaction (where reduction
takes place).
For example,
Redox reaction: Zn + Cu2+→ Zn2+ + Cu
Oxidation half reaction: Zn → Zn2+ + 2e−
Reduction half reaction: Cu2+ + 2e−→ Cu
OXIDIZING AGENTS AND REDUCING AGENTS
Oxidizing agents are those substances, which can oxidize some other substances. To do so, it will have to gain electron (decrease in oxidation state) and hence it will be reduced. Similarly the reducing agent will give electrons and will be oxidised. So the substances undergoing oxidation are reducing agent and the substances undergoing reduction are oxidizing agents. In the reaction, Zn + Cu 2+ → Zn2+ + Cu, Zn is oxidised to Zn2+, so Zn is reducing agent (it is reducing Cu2+ to Cu) and Cu2+ is reduced to Cu, so Cu+2 is oxidizing agent (it is oxidizing Zn to Zn2+).
Guidelines for the identification of Oxidizing and Reducing Agents
- If an element is in its highest possible oxidation state in a compound, it can function as an oxidizing agent, e.g. KMnO 4 , K 2 Cr 2 O 7 , HNO 3 , H 2 SO 4 , HClO 4 etc.
- If an element is in its lowest possible oxidation state in a compound, it can function as a reducing agent, e.g. H 2 S, FeSO 4 , Na 2 S 2 O 3 , SnCl 2 etc.
- If an element is in its intermediate oxidation state in a compound, it can function both as an oxidizing agent as well as reducing agent, e.g. H 2 O 2 , H 2 SO 3 , HNO 2 , SO 2 etc.
- If highly electronegative element is in its higher oxidation state in a compound, that compound can function as a powerful oxidizing agent, e.g. KClO 4 , KClO 3 , KIO 3 etc.
- If an electronegative element is in its lowest possible oxidation state in a compound or in free state, it can function as a powerful reducing agent, e.g. I − , Br − , N 3− etc.
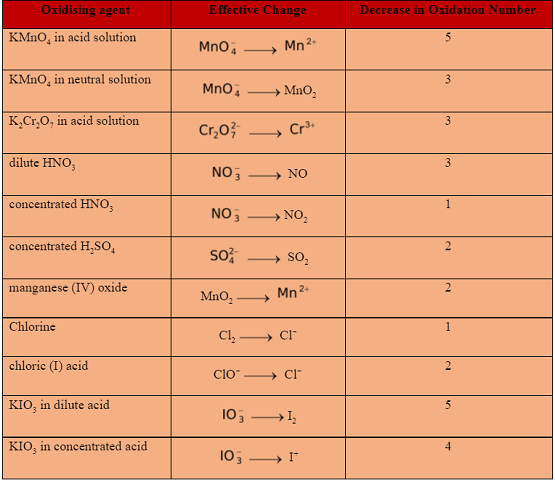
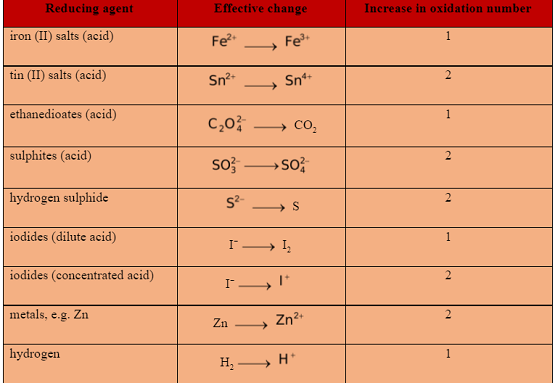
COMMON OXIDISING AND REDUCING AGENTS
The functioning of some common oxidising and reducing agents is summarised below.