Electrochemical Cells
Electrochemistry of Class 12
Electrochemical Cells
Electrochemical cells are the cells in which chemical energy is converted into electrical energy. This means that chemical reactions produce electric current.
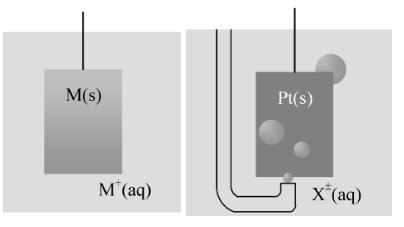
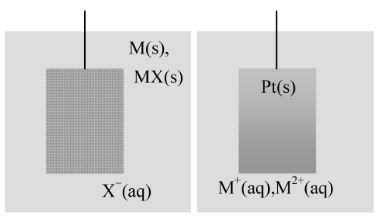
An electrochemical cell consists of two electrodes, (metallic conductors) in contact with an electrolyte, an ionic conductor (which may be a solution, a liquid, or a solid). An electrode and its electrolyte comprise an electrode compartment. The two electrodes may share the same electrolyte. The various kinds of electrode are summarized in the following table and illustrated in the accompanying figure.
Different types of electrodes
|
S. No. |
Electrode type |
Designation |
Half-reaction |
|
(a) |
Metal−metal ion |
M + (aq) | M(s) |
M + (aq)+e− → M(s) |
|
(b) |
Gas−ion electrode |
X + (aq) | X2(g)⏐ Pt(s) |
X + (aq)+e− → ½X2(g) |
|
X−(aq) ⏐X2(g) | Pt(s) |
½X 2 (g)+e− → X−(aq) |
||
|
(c) |
Metal−insoluble salt−anion |
X−(aq) | MX(s)⏐M(s) |
MX(s)+e−→M(s)+X−(aq) |
|
(d) |
Redox electrode |
M 2+ (aq) , M + (aq) | Pt(s) |
M 2+ (aq)+e− → M + (aq) |
(a) (b)
(c) (d)
In a given electrochemical cell, combination of any of the two electrodes can be used. The cell may even contain same types of electrodes with different concentration of electrolytes.
For example, Pt(s) | X
2
( 1 atm) |
X
+
(c1 M) ||
X
+
(c
2
M) | X
2
( 1 atm) | Pt(s)
When an ‘inert metal’ is part of the cell, it is present to act as a source of electrons, but takes no other part in the reaction other than acting as a catalyst for it. If the electrolytes are different, the two compartments may be joined by a salt bridge, which is a concentrated electrolyte solution in agar−agar jelly that completes the electrical circuit and enables the cell to function. A galvanic cell is an electrochemical cell that produces electricity as a result of the spontaneous reaction occurring inside it.
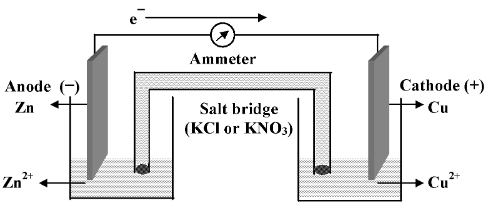
One of the simplest electrochemical cell, called Daniel cell is shown in the given figure. It consists of two redox couples such as Cu 2+ | Cu at one end and Zn 2+ | Zn at the other end. Since Zn has a higher oxidation potential than Cu, it gets oxidized to Zn 2+ at anode. At the same time Cu 2+ gets reduced to Cu at the cathode.
|
|
The salt bridge contains a highly soluble electrolyte (like KCl, NH 4 NO 3 , NH4Cl, KNO 3 etc) in which ionic mobilities of cation and anion are of comparable order. The zinc rod which has the electron left by the zinc (that got oxidized) becomes negatively charged while the Cu rod which lost the electrons to Cu 2+ becomes positively charged. As these two half cells are connected through connecting wires and an ammeter, electric current flows which is indicated by a deflection in ammeter showing that a chemical reaction is occuring in the cell. During the course of reaction, zinc rod gets dissolved and copper is deposited on the copper rod. Thus the concentration of the anode solution increases while that of cathode solution decreases. The flow of electrons occurs from the zinc rod to copper rod in the external circuit. The current flow is continuous as long as the electrical connections and the salt bridge are maintained and sufficient amount of reactant solution remains.
Now let us analyze what happens in each compartment more carefully. We note that electrons flow from the zinc rod through the external circuit, and that zinc ions are produced as the zinc rod dissolves. We can summarize these observations by writing Zn(s)
→ Zn
2+
(aq) + 2e−
(at the zinc rod). Also we observe that electrons flow to the copper rod as cupric ions leave the solution and metallic copper is deposited. We can represent these occurrences by
Cu
2+
(aq) + 2e−
→
Cu(s) (at the copper rod).
In addition, we must examine the purpose of the salt bridge. Since zinc ions are produced as electrons leave the zinc electrode, this tends to produce a net positive charge in the left compartment. After sometime the solution would become so positively charged (due to passing of Zn 2+ in the solution) that solution would repel any further Zn 2+ coming in the solution. Similarly, no Cu 2+ would get reduced at the cathode due to repulsion from the Cu rod. Thus the net charge accumulating in the two compartments would immediately stop the electron flow through the external circuit, and the oxidation reduction reaction would stop. Thus, while the salt bridge does not participate chemically in the cell reaction, it is necessary if the cell is to operate. The salt bridge keeps the solution neutral by passing appropriate amounts of cations or anions to the two half cells (compartments). Thus the purpose of the salt bridge is to prevent any net charge accumulation in either compartment.
Cell Notation
- Anode is written on the left of the notation and cathode is written on the right.
- In the notation for cells, phase boundaries are denoted by vertical bar or slash.
- Concentration of the electrolytes in the anode and cathode must be written in parenthesis.
- In case of a gas, the partial pressures are to be indicated in atm or mm Hg.
- A comma is used to separate two chemical species in the same solution.
- A double vertical line || denotes an interface for which it is assumed that the liquid junction potential (potential developed between the electrolyte and the electrode) has been eliminated, i.e. a salt bridge is present.
- EMF of the cell is written on the extreme right of the representation.
For example,
(i) Zn(s) | ZnSO 4 (aq) || CuSO 4 (aq) | Cu(s) ; Ecell
(ii) Pt | H
2
(p
1
) | HCl (c M) | AgCl(s) | Ag ;
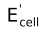
(iii) Pt |
Fe
2+
(c1),
Fe
3+
(c
2
) ||
Ag
+
(c) | Ag ;
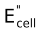
[Note: In some cell representations, the salt bridge is not indicated which means that the electrolyte is common to both anode and cathode compartment].