
Hard And Soft Water
Hydrogen of Class 11
Hard And Soft Water
Water is classified into categories depending upon its behaviour towards soap solution.These are: soft water and hard water.
- Soft Water: Water which produces lather with soap solution readily is called soft water. Distilled water and rain water are common examples of soft water.
- Hard Water: Water which does not produce lather with soap solution easily is called hard water. Sea water, tap water are common examples of hard water.
Cause of Hardness of water
Hardness of water is due to the dissolved impurities of the salts like bicarbonates, chlorides and sulphates of calcium and magnesium. Water gets contaminated by these salts when it passes through the ground and rocks. Hard water does not produce lather with soap solution readily because the cations
 present in hard water react with soap (which is a mixture of sodium slats of higher fatty acids like stearic acid, palmitic acid, oleic acid, etc) to form a precipitate of calcium and magnesium salts of fatty acids.
present in hard water react with soap (which is a mixture of sodium slats of higher fatty acids like stearic acid, palmitic acid, oleic acid, etc) to form a precipitate of calcium and magnesium salts of fatty acids.
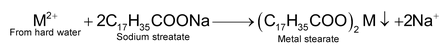
(M = Ca or Mg)
Thus, no lather is produced until all the calcium and magnesium ions have been precipitated. This leads to the consumption and hence, wastage of lot of soap. Hard water is, therefore, not fit for washing purposes
Related Topics
Types of Hardness
The hardness of water is of two types: Temporary Hardness and Permanent Hardness.
(i) Temporary hardness.
It is due to the presence of soluble bicarbonates of calcium and magnesium. Such water is also said to posses carbonate hardness. The term temporary indicates that most of the hardness can be removed by simply boiling the water. The bicarbonates of calcium and magnesium are formed in water by dissolution of carbonates of calcium and magnesium in the presence of atmospheric carbon dioxide.
(ii) Permanent hardness.
It is due to the presence of chlorides and sulphates of calcium and magnesium. Such water is also said to posses non-carbonate hardness. The term permanent indicates that type of harness can not be removed by mere boiling of water.
Softening of Hard Water
The process of removal of metallic ions
 responsible for hardness of water is known as softening of water. A number of methods are available to soften water depending upon the nature of dissolved mineral salts as described below:
responsible for hardness of water is known as softening of water. A number of methods are available to soften water depending upon the nature of dissolved mineral salts as described below:
Removal of Temporary Hardness
Temporary hardness can be removed by the following methods:
(i) Boiling
Temporary hard water is taken in large boilers and boiled for about fifteen minutes. Consequently, the bicarbonate of calcium and magnesium present in the water decompose into their insoluble carbonates which settle at the bottom of the tank as precipitate which are removed by filtration or decantation.
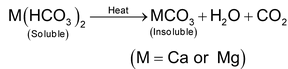
(ii) Calcium hydroxide (or Clark’s) method
Calculated quantity of lime (calcium hydroxide) is added to temporary hard water. The soluble bicarbonates are converted into insoluble carbonates which settle at the bottom of the tank and are removed by filtration.
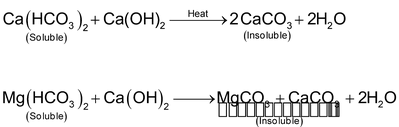
Removal of Permanent Hardness
Permanent hardness of water can be removed by the following methods:
1. By chemical additives
(a) Addition of washing soda.
In this method,
 ions can be precipitated by the addition of calculated amount of washing soda
ions can be precipitated by the addition of calculated amount of washing soda
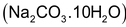

(b) Addition of sodium polymetaphosphate (calgon process)
In this method
 ions are rendered ineffective by the addition of sodium polymetaphosphate. The trade name of which is calgon (meaning calcium gone). The general formula of sodium polymetaphosphate is
ions are rendered ineffective by the addition of sodium polymetaphosphate. The trade name of which is calgon (meaning calcium gone). The general formula of sodium polymetaphosphate is
 , where the value of n is sometime as large as 1000. However, commonly, calgon is represented by sodium hexametaphosphate
, where the value of n is sometime as large as 1000. However, commonly, calgon is represented by sodium hexametaphosphate
 .The
.The
 of hard water reacts with calgon to form soluble complexes.
of hard water reacts with calgon to form soluble complexes.
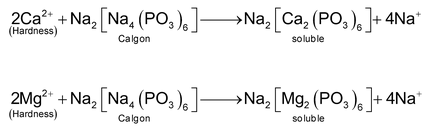
The complexes of calcium and magnesium so formed remain dissolved in water but they do not cause hindrance in the formation of lather. This is because calcium and magnesium ions are not free to react with soap but these have been tied up in stable complexes. This is also known as sequestraction of
 ions.
ions.
2. Ion Exchange method
This is a modern method of softening of water. In this method, the ions present in the hard water are exchanged for less damaging ions from the exchangers. There are two main types of ion-exchanger as:
(a) Inorganic cation exchangers (Permutit Method)
These are complex inorganic salts like hydrated sodium-aluminum silicate
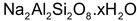 which have interesting property of exchanging cations such as calcium and magnesium ions in hard water for sodium ions. These complex salts are known as zeolites which can be either a naturally occurring or an artificially synthesized substances. Their technical name is permutit. For artificial synthesis of permutit, a mixture of soda ash
which have interesting property of exchanging cations such as calcium and magnesium ions in hard water for sodium ions. These complex salts are known as zeolites which can be either a naturally occurring or an artificially synthesized substances. Their technical name is permutit. For artificial synthesis of permutit, a mixture of soda ash
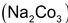 , sand
, sand
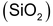 and alumina
and alumina
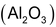 is fused together. The product is washed with water to remove soluble impurities leaving behind a porous mass of permutit.
is fused together. The product is washed with water to remove soluble impurities leaving behind a porous mass of permutit.
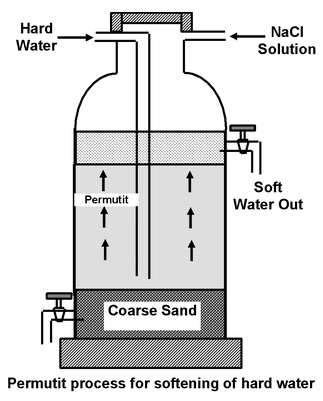
The permutit is loosely packed in a big tank over a layer of coarse sand. Hard water is introduced into the tank from the top. Water reaches the bottom of the tank and then slowly rises through the layer of permutit in the tank. The cations present in hard water are exchanged for sodium ions.
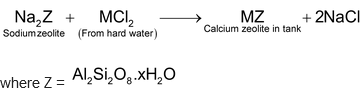 (M = Ca or Mg)
(M = Ca or Mg)
As the process is continued, zeolites gets exhausted because of its conversion into calcium and magnesium zeolite. The exhausted resin is regenerated allowing about 10% solution of sodium chloride to percolate through it when the following reaction occurs.
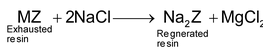 (M = Ca or Mg)
(M = Ca or Mg)
The regenerated or reactivated resin can be used again and again for a quite longer period.
(b) Organic ion exchangers
These are complex organic molecules having giant hydrocarbon frame work with either acidic group (-SO 3 or -COOH) or basic group (OH - or NH 2 - ) attached to them. The resins with acidic group are capable of exchanging the H + ions for the cations and are called cation exchangers. They are represented as H + - resin. The resins with basic group are capable of exchanging their OH - or NH 2 - ions for other anions and are called anion exchangers. They are represented as HO - -resin.
Method of Removal of Hardness By Organic Exchangers
First of all, the hard water is passed through a bed of cation exchange resin. The cations present in hard water are exchanged with H + of resin as:
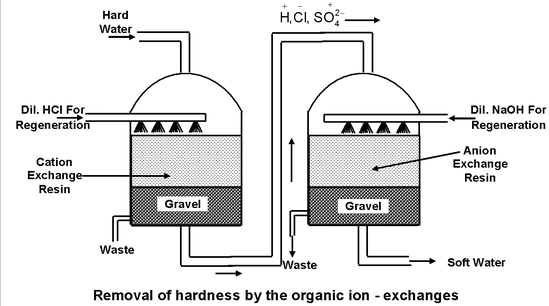
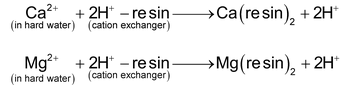
The water which comes out of the bottom of first tank is richer in H + ions.
This water is then passed through a bed of anion exchange resin where anions contained in water are exchanged with OH- ions as
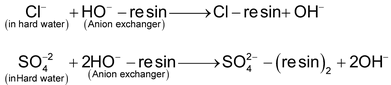
Therefore, the H + ions (formed in the first tank) combine with the OH- ions (formed in the second tank) to produce water.
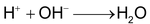
Thus, water obtained by this method is free from all types of cations as well as anions. It is known as deionised or demineralised water.
This method is particularly suitable for obtaining pure water for laboratory purposes.
Regeneration of resins
The exhausted resin in the first tank is regenerated by treatment with moderately concentrated hydrochloric or sulphuric acid.
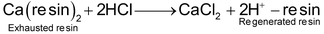
Similarly, the exhausted resin in the second tank is regenerated by treatment with moderately concentrated solution of sodium hydroxide.
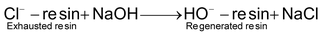
Degree of Hardness of Water
Degree of hardness of water is defined as number of parts of mass of CaCO 3 (Calcium carbonate), equivalent to various calcium and magnesium salts present in one million parts by mass of water. It is expressed in ppm (parts per million).
It may be noted degree of hardness up to 100 – 150 ppm in water required for our daily needs such as cooking, bathing, washing of clothes, etc, is tolerable. But if degree of hardness exceeds this limit, then water is not suitable for domestic use.




