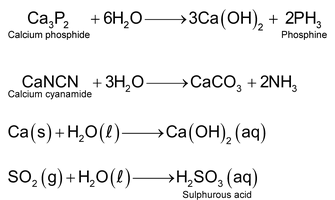
Water
Hydrogen of Class 11
Water (H2O) is an important hydride of oxygen, which is principal (about 75%) constituent of earth’s surface. It is most abundant, omnipresent and easily obtainable of all chemical compounds. It is a significant component of animal and vegetable matter and play a vital role in their process. It ranks next to oxygen in importance of out existence. It constitutes about 65% of human body and about 95% by weight of some plants. It can be easily transformed from liquid to solid or from liquid to gaseous states and vice versa.
Distribution of Water
The distribution of water over the earth’s surface is not uniform
The desert regions have no permanent surface water while the oceans cover about 78% of the earth’s surface. They contain 97% of the available water. Out of the total surface water only 2.7% is fresh water and rest is locked in polar ice caps, glaciers or under the ground and is not readily available.
Structure and Aggregation of Water Molecules
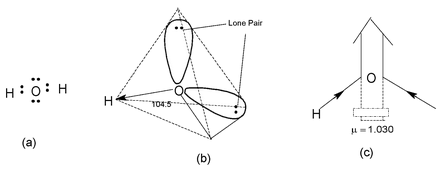
In water molecule, the two H atoms are bonded to O atoms by two covalent bonds. The oxygen atom assumes sp3 hybrid state. Each of the covalent bonds are formed by the axial overlap of 1s-orbital of H atom and sp3 hybrid orbital of O atom. The two bond pairs and two lone pairs of electrons around oxygen atom assume tetrahedral arrangement. Consequently, the H2O molecules has a bent structure. Due to relativity greater repulsive interactions of lone pairs the bond angle around the O atom decreases from 109.28′ to 104.5′ as shown in the figure.
Polar nature of H2O
Since water molecule has bent shape, therefore bond moments of two
O – H bond causes the molecule to behave as permanent electrical dipole. The dipole moment of H2O molecule has been found to be 1.84 D which confirms its polar nature, as shown in the figure.
Aggregation of Water Molecules
In gaseous state, the individual covalent molecules H2Oexist as such. However in liquid state, large aggregates of H2Ounits are formed because of their association through intermolecular hydrogen bonds as shown below
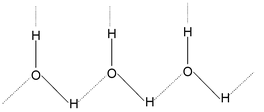
The extent of association, however, depends upon the conditions of temperature and pressure.
The intermolecular hydrogen bonding is responsible for the abnormally high freezing point, boiling point, heat of fusion and heat of vaporization as compared to the hydrides of the other elements of oxygen family.
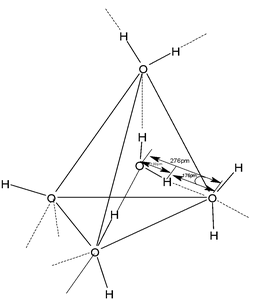
In ice, a solid state of water, each H2O molecule is tetrahedrally surrounded by four neighboring H2O molecules with their oxygen atoms occupying the corners of tetrahedron. There are four H atoms around each O atom. Two of the four H atoms are bonded by covalent bonds (bond length 100 pm) whereas the other two are linked through hydrogen bonds (176 pm) as shown in the figure. This gives highly three dimensional structure having large vacant spaces, which may be compared to open cage.
Due to open cage like structure, ice has a relatively larger volume for a given mass of liquid water. Consequently, density of ice is less than water and it floats over water.
As the temperature is raised beyond 273 K, open cage like structure dismentalling due to cleavage of some H-bonds and ice melts. The breaking of H-bond causes aggregation of H2O molecules to have closer resulting in the decrease in volume and thereby increase in density. This continues till 277 K. When the density becomes maximum. Beyond 277 more and H-bonds cleaves and expansion of liquid water starts occurring due to increased K.E. of molecules and the density again starts decreasing however, it remains higher than ice. Hence density of water is maximum at 277 K.
|
This property of maximum density at 277 K is a boon for the survival of aquatic animals during winter months because when the upper layer of sea water freezes. The frozen water does not sink to the bottom but keeps floating at the surface due to its lesser density. This provides thermal insulation to the water below it. It is very interesting to note that nine different crystalline forms of ice have been found to be exist under different pressure conditions. Each one has different melting point. At one bar pressure ice has normal hexagonal form. However at low temperature it adopts cubic form. |
|
Properties of Water Physical properties
Water has some unique features which arise due to intermolecular H-bonding. Some important physical constants of water are given in table.
|
Property |
Constant |
|
Molecular mass |
18.015 |
|
Melting Point (K) |
273.2 |
|
Boiling Point (K) |
373.2 |
|
Temperature of maximum Density (K) |
277.1 |
|
Maximum Density (g cm-3) |
1.000 |
|
Density (g cm-3) |
0.997 |
|
Heat of Vaporisation (373 K)(kJ/mol-1) |
40.66 |
|
Heat of Fusion (kJ/mol-1) |
6.01 |
|
Specific Heat (Jg-1K-1) |
4.177 |
|
Ionization Constant [H+][OH-](mol2L-2) |
1.008 x 10-14 |
|
ΔH°f (kJmol-1) |
-285.9 |
Some of the important physical properties are discussed below:
(i) The freezing point, boiling point, heat of fusion and heat of vapourization water are abnormally higher than those of the hydrides of the other elements of the same group (16) such as H2S,H2Se,H2Te etc. This is due to the presence of intermolecular hydrogen bonding in H2O molecules which is, however absent among the molecule of H2S,H2Se,H2Te etc.
(ii) Water has a higher specific heat, thermal conductivity and surface tension than most other liquids. These properties allow water to play a vital role in the biosphere. For example, the high heat of vapourization and the high heat capacity of water are responsible for moderation of the climate and body temperature of living organisms.
(iii) Water because of its high dielectric constant (78.39) has the ability to dissolve most of the inorganic (ionic) compounds and its, therefore, regarded as a universal solvent. Whereas solubility of ionic compounds takes place due to ion-dipole interactions (i.e. solvation of ions), the solubility of covalent compounds such as alcohols, amines, urea, glucose, sugar etc, takes place due to the tendency of these molecules to form hydrogen bonds with water.
Chemical Properties of Water
1. Action towards litmus
Pure water is neutral to litmus.
2. Decomposition
Water is quite stable and does not dissociate at high temperature. The dissociation into its elements is only 0.02 % even at 1500K.
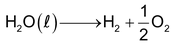
The small conductivity of pure water reveals its dissociation into H3O+ and OH- ion.
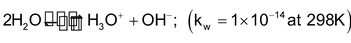
3. Acid-base reactions
Water is amphoteric substance because it can act as acid as well as base as shown -
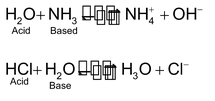
However, the pH of water at 25° is 7 and its is neutral towards litmus.
4. Hydrolytic reactions
Water can hydrolyse many non-metallic oxides, halides and also some metallic phosphides, carbides and nitrates.