
Cupric Sulphate Or Blue Vitriol
Inorganic Compound of Class 12
Cupric Sulphate Or Blue Vitriol
This compound is generally called copper sulphate or Nila Thotha. In the laboratory, it is prepared by the action of cupric oxide, carbonate or hydroxide on dilute sulphuric acid followed by evaporation and crystallization.
CuO + H
2
SO
4
→ CuSO
4
+ H
2
O
CuCO
3
+ H
2
SO
4
→ CuSO
4
+ H
2
O + CO
2
Cu(OH)
2
+ H
2
SO
4
→ CuSO
4
+ 2H
2
O
Manufacture
On a large scale copper sulphate is obtained by boiling copper turning with concentrated sulphuric acid. Blue solution of copper sulphate is formed.
Cu + 2H
2
SO
4
→ CuSO
4
+ SO
2
+ 2H
2
O
It is also obtained by treating copper scrapping in hot dilute sulphuric acid in presence of air.
2Cu + 2H
2
SO
4
→ 2CuSO
4
+2H
2
O
Copper scrap or copper turnings are packed in a lead lined vertical tower. Dilute sulphuric acid is sprayed at the top and percolates through copper chips. A current of air and steam is blown from below. Copper dissolves in dilute sulphuric acid in the presence of air to give a solution of copper sulphate which is collected at the base. This solution of copper sulphate contains a lot of dilute acid and is recirculated in the tank by means of a pump to get a concentrated solution which is led into crystallization tanks. On cooling, crystals of pentahydrate copper sulphate. CuSO
4
.5H
2
O are deposited.
Properties
(i) Copper sulphate forms deep blue crystals of the composition CuSO 4 .5H 2 O. Copper sulphate is fairly soluble in water.
(ii)Action of heat: On heating copper sulphate loses its water of crystallization. On heating to 100°C it gives CuSO
4
.H
2
O and when heated to 230°C it becomes white anhydrous CuSO
4
.
The white anhydrous copper sulphate decomposes at about 720°C to give cupric oxide and sulphur trioxide.
CuSO
4
.5H
2
O
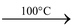 CuSO
4
.H
2
O
CuSO
4
.H
2
O
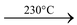 CuSO
4
CuSO
4
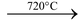 CuO + SO
3
CuO + SO
3
blue Pale blue colourless (amorphous)
(iii)Copper sulphate gives all the general reactions of sulphates.
(iv)Action of potassium iodide: Copper sulphate gives white cuprous iodide with potassium iodide.
2CuSO
4
+ 4KI → 2K
2
SO
4
+2CuI + I
2
(v) Action of alkalies: Alkalies produce a pale blue precipitate of copper hydroxide.
CuSO
4
+ 2NaOH → Cu(OH)
2
+ Na
2
SO
4
Pale blue ppt.
(vi) Action of NH
4
OH: With ammonium hydroxide, precipitate of Cu(OH)
2
is first produced but it dissolves in excess of it forming a bright blue solution, the colour being due to the formation of cuprammonium ion, [Cu(NH
3
)
4
]
2+
CuSO
4
+ 2NH
4
OH → Cu(OH
2
+ (NH
4
)
2
SO
4
small amount ppt.
CuSO
4
+ 4NH
4
OH → Cu(NH
3
)
4
SO
4
+ 4H
2
O
large amount Cuprammonium
sulphate
(vii) Action of potassium ferrocyanide: Reddish brown precipitate of cupric ferrocyanide is formed.
2CuSO 4 + K 4 [Fe(CN) 6 → 2K 2 SO 4 + Cu 2 [Fe(CN) 6 ]
Reddish brown ppt.
(viii) Action of potassium sulphocyanide: Cupric sulphocyanide is formed.
CuSO
4
+ 2KCNS → K
2
SO
4
+ Cu(CNS)
2
Cupric sulphocyanide
(ix)Action of KCN: With KCN it gives a yellow ppt. of cupric cyanide which decomposes to give cuprous cyanide and cyanogens. Cuprous cyanide dissolves in excess of KCN to give potassium cuprocyanide.
CuSO
4
+ 2KCN → Cu(CN)
2
+ (CN)
2
K
2
SO
4
2Cu(CN)
2
→ Cu
2
(CN)
2
+ (CN)
2
Cupric Cuprous Cyanogen
cyanide cyanide
Cu
2
(CN)
2
+ 6KCN →2K
3
Cu(CN)
4
Potassium cuprocyanide
(x) Addition of electropositive metals: Electropositive metals like zinc or iron precipitates copper from a solution of copper sulphate.
CuSO
4
Fe → FeSO
4
+ Cu
(xi) Action of H
2
S: With H
2
S it gives a black ppt. of copper sulphide.
CuSO
4
+ H
2
S → CuS + H
2
SO
4
Black ppt.
Uses
- In electroplating in electrotyping and in the purification of copper.
- In agriculture as a germ killer and fungicide. Bordeaux mixture is copper sulphate mixed with lime and is extensively used for dusting vines and potatoes.
- In calico−printing, dyeing and the preparation of other compounds of copper.
- In analysis (as anhydrous salt).
- In medicine as an antiseptic.




