Functional Group
IUPAC & GOC of Class 11
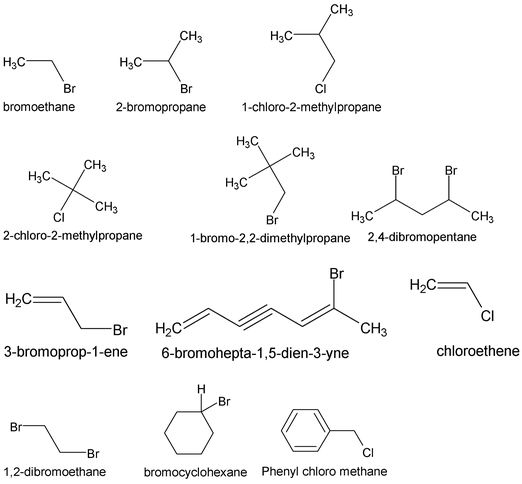
1. Alkyl Halide
The alkyl halides have the general formula C n H 2n+1 X or RX, where X denotes fluorine, chlorine bromine or iodine.
2. Alcohols
General formula [C n H 2n+1. OH], IUPAC name is alkanol.
For the simpler alcohols the common names, are most often used. These consist simply of the name of the alkyl group followed by the word alcohol. For example:
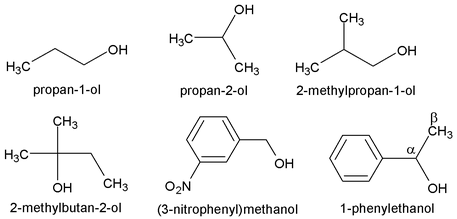
We should notice that similar names do not always mean the same classification; for example, isopropyl alcohol is a secondary alcohol, whereas isobutyl alcohol is a primary alcohol.
Finally, there is the most versatile system, the IUPAC. The rules are:
1. Select as the parent structure the longest continuous carbon chain that contains the -OH group; then consider the compound to have been derived from this structure by replacement of hydrogen by various groups. The parent structure is known as ethanol, propanol, butanol, etc., depending upon the number of carbon atoms; each name is derived by replacing the terminal -e of the corresponding alkane name by -ol.
2. Indicate by a number the position of the -OH group in the parent chain, generally using the lowest possible number for this purpose.
3. Indicate by numbers the positions of other groups attached to the parent chain.
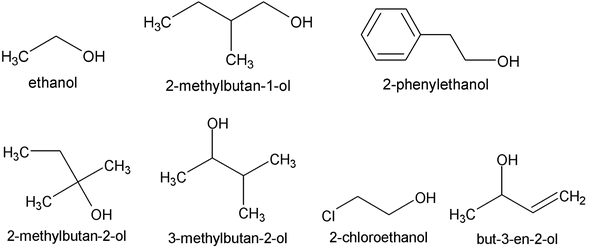
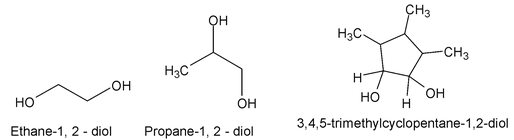
Alcohols containing two hydroxyl groups are called glycols. They have both common names and IUPAC names.
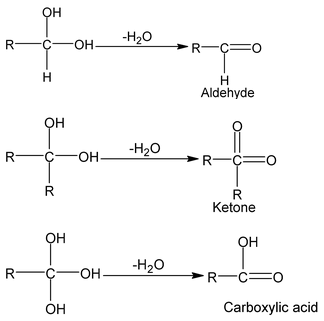
Note:
2 or 3 OH group can not present on same carbon atom, decomposes to give aldehyde/ketone or carboxylic acid respectively.
3. Ether (R − O − R]
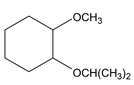
Ethers are compounds in which two C atoms are connected to a single O atom. In IUPAC nomenclature, name one of the alkyl group plus the O atom (RO−) as an alkoxy and comes as a prefix to the parent hydrocarbon. (Oxygen is to be counted with least number of carbon atom)
IUPAC name of ether is alkoxy alkane
|
CH 3 OCH(CH 3 ) 2 2 – Methoxy propane (isopropyl methlyl ether) |
|
|
|
1 – isopropoxy – 2 – methaoxy – cyclohexane |
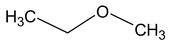 Methoxy ethane
Methoxy ethane
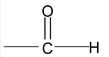
4. Aldehydes
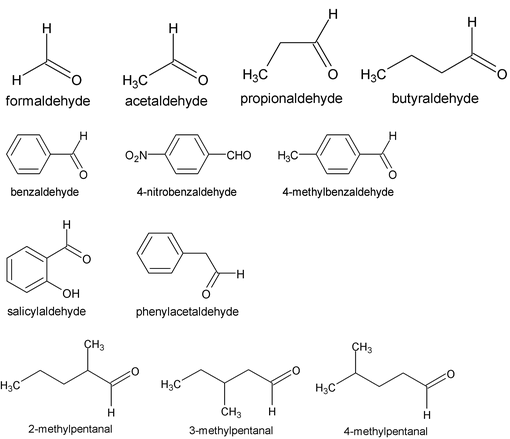
IUPAC names the longest continuos chain including the C of – CH = O and replaces – e of the alkane name by the suffix – al i.e. alkanal. The C of CHO is number 1. For compounds with two – CHO groups, the suffix – dial is added to the alkane name. When other functional groups have naming priority, – CHO is called formyl.
Common names replace the suffix –ic (–oic or – oxylic) and the word acid of the corresponding carboxylic acids by – aldehyde. Locations of substituents on chains are designated by Greek letters e.g.
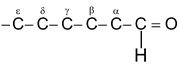
The terminal C of a long chain is designated ω (omega)
The IUPAC names of aldehydes follow the usual pattern. The longest chain containing the –CHO group is considered the parent structure and named by replacing –e of the corresponding alkane by –al. The position of the substituent is indicated by a number, the carbonyl carbon always being considered C-1. Here, as with the carbonyl acids, the C-2 of the IUPAC name corresponds to alpha of the common name.
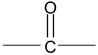
5. Ketones
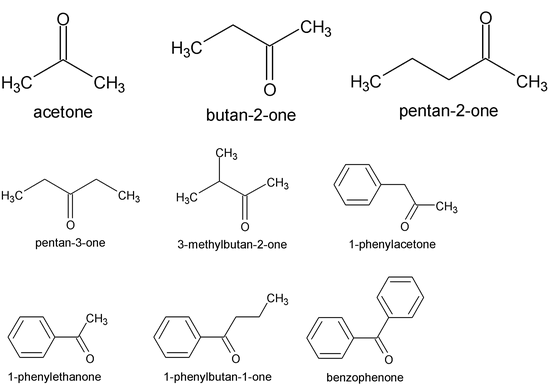
Common names use the names of R or Ar as separate words, along with the word ketone. The IUPAC system replaces the –e of the name of the longest chain by the suffix –one. In molecules with functional groups such as – COOH, that have a higher naming priority, the carbonyl group is indicated by the prefix keto or oxo. Thus, CH 3 COCH 2 CH 2 COOH is 4-ketopentanoic acid.
The simplest aliphatic ketone has the common name acetone. For most other aliphatic ketones we name the two groups that are attached to carbonyl carbon and follow these names by the word ketone. A ketone in which the carbonyl group is attached to a benzene ring is named as phenone, all illustrated below. The positions of various groups are indicated by numbers.
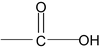
6. Carboxylic acid
(i) Common or Trivial names:
The names of lower members are derived from the Latin or Greek word that indicate the source of the particular acid.
|
Formula |
Source |
Common or trivial names |
|
HCOOH |
Red ant (Latin ant – formica) |
Formic acid |
|
CH 3 – COOH |
Vinegral (Latin vinegar acetum) |
Acetic acid |
|
CH 3 – CH 2 – COOH |
Proton-pion
|
Propionic acid |
(ii) Derived System:
Monocarboxylc acid may be named as alkyl derivative of acetic acid.
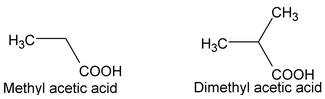
(iii) IUPAC System:
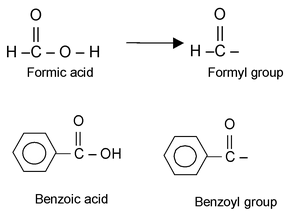
Acids are named as alkanoic acids. The name is derived by replacing ‘e’ of the corresponding alkane by oic acid.
HCOOH Methanoic acid
CH 3 COOH Ethanoic acid
CH 3 CH 2 COOH Propanoic acid
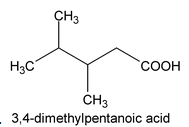
In case of substituted acids, the longest chain including carboxyl group is selected and numbering is done from the carbon of carboxylic group.
Example:
7. Derivative of Carboxylic acid
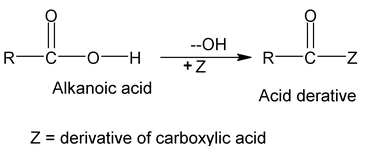
Acyl Group
(i) Naming Acyl Groups- Acid halide and Anhydrides
The group obtained from a carboxylic acid by the removal of the hydroxyl portion is known as an acyl group. The name of an acyl group is created by changing the - ic acid at the end of the name of the carboxylic acid to –yl, examples:
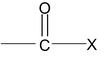
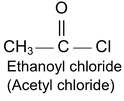
(a) Acid Halide
IUPAC name of acid halide is alkanoyl halide.
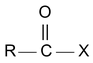
where X may be Cl, Br, I
Acid chlorides are named systematically as acyl chlorides.
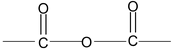
(b) Acid anhydride
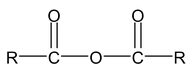
An acid anhydride is named by substituting anhydride for acid in the name of the acid from which it is derived.
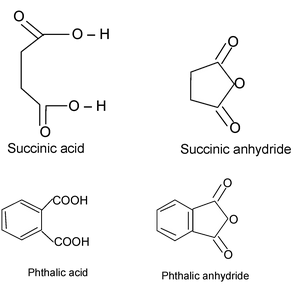
IUPAC name of acid anhydride is alkanoic anhydride.
Example:
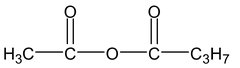
Butanoic, ethanoic anhydride
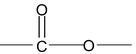
(ii) Naming Salts and Esters
General formula of ester RCOOR′
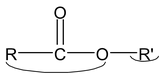
IUPAC name: Alkyl alkanoate
Example:
CH 3 COOC 2 H 5 (Ethyl ethanoate)
The name of the cation (in the case of a salt) or the name of the organic group attached to the oxygen of the carboxyl group (in the case of an ester) precedes the name of the acid.
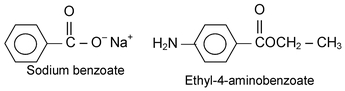
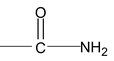
(iii) Name of Amides and Imides
The names of amides are formed by replacing –oic acid (or –ic acid for common names) by amide or –carboxylic acid by carboxamide.
IUPAC name of acid amide is alkanamide
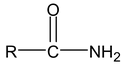
Example:
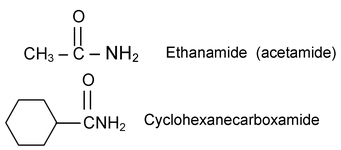
If the nitrogen atom of the amide has any alkyl groups as substitutents, the name of the amide is prefixed by the capital letter N; to indicate substitution on nitrogen, followed by the name(s) of alkyl group(s).
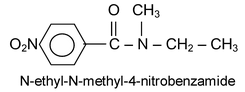
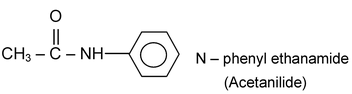
If the substituent on the nitrogen atom of an amide is a phenyl group, the ending for the name of the carboxylic acid is changed to anilide
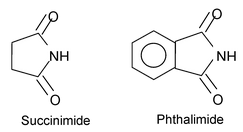
Some dicarboxylic acids form cyclic amides in which two acyl groups are bonded to the nitrogen atom. The suffix imide is given to such compounds.
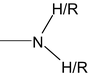
8. Amine
Nomenclature of amines
Nomenclature of amines is quite simple. Aliphatic amines are named by naming the alkyl group (or) groups attached to nitrogen , and following that by the word amine.
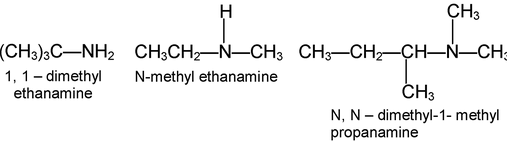
More complicated amines are often named as prefixing amino - (or-N-methylamino -, N-N, diethyl amino -, etc) to the name of the parent chain.
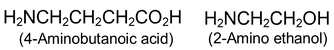
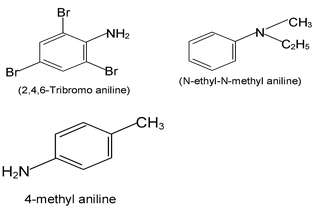
Aromatic amines - those in which nitrogen is attached to an aromatic ring - are generally named as derivatives of the simplest aromatic amine, aniline.
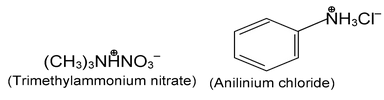
Salts of amines are generally named by replacing - amine by - ammonium (or - aniline by - anilinium), and adding the name of the anion.
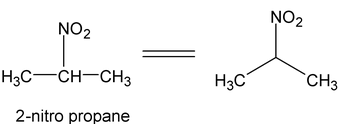
9. Nitro Alkane
General formual C n H 2n+1 .NO 2
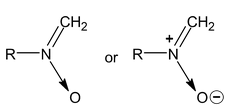
Nitro alkane
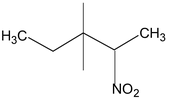
Example:
|
|
|
|
|
2 nitro 3, 3 dimethyl pentane |
Alkyl nitrites
Common name is alkyl nitrite there is no IUPAC name of nitrite.
R – O – N = O Alkyl nitrite
CH 3 CH 2 – O NO Ethyl nitrite
10. Alkyl Cyande
R – C ≡ N Alkane nitrile
Example:
CH3 – C ≡ N Ethane nitrile
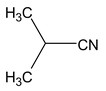
2 methyl propane nitrile
Alkyl iso cyanide RNC
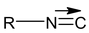
Alkyl iso nitrile or alkyl iso cyanide.
There is no specific IUPAC name for alkyl iso cyanide or isonitrile.
CH 3 NC Ethyl isonitrile
When a compound contains more than one functional group, the numbering and the suffix in the name of multifunctional compound are determined by nomenclature priority.
Preference Order
|
Class |
Formula |
Suffix (if present as a functional group) |
Prefix (if present as a substituent) |
|
Carboxylic acid |
|
−oic acid |
Carboxy |
|
Acid ahydrides |
|
−oic anhydride |
|
|
Eaters |
|
Alkyl alkanoate |
Alkoxycarbonyl |
|
Acyl halides |
|
−oyl halide |
Haloalkanoyl |
|
Amides |
|
-amide |
Carbamoyl |
|
Nitriles |
− C ≡ N |
Nitrile |
Cyano |
|
Aldehydes |
|
−al |
Formyl |
|
Ketones |
|
−one |
Oxo |
|
Alcohols |
−OH |
−ol |
Hydroxy |
|
Amines |
|
Amine |
Amino |
|
Etehrs |
− O − |
Alkoxy alkane |
−oxy |
|
Alkenes |
|
−ene |
Alkenyl |
|
Alkynes |
− C ≡ C − |
-yne |
Alkynyl |
|
Halides |
−X |
− |
Halo |
|
Nitro |
−NO 2 |
− |
Nitro |
|
Alkanes |
|
−ane |
Alkyl |
|
Benzene |
|
Benzene |
Phenyl |