
Osmosis And Osmotic Pressure
Liquid Solution of Class 12
Osmosis And Osmotic Pressure
There is a natural tendency of solutes in a solution to diffuse from a higher concentration to a lower concentration so as to bring about a uniform distribution throughout.
Certain membranes allow solvent molecules to pass through them but not solute molecules, particularly not those of large molecular weight. Such a membrane is called semipermeable and might be an animal bladder, a vegetable tissues or a piece of cellophone.
Osmosis is the phenomenon of solvent flow thorough a semi permeable membrane to equalize the solute concentrations on both sides of the membrane.
Osmotic pressure is a colligatvie property of a solution equal to the pressure that, when applied to the solution just stop osmisos. It is denoted by π. The osmotic pressure π of a solution is related to the molar concentration of solute M.
π = MRT, Where R is the gas constant and T is absolute temperature.
Van’t Hoff equation for dilute solution is similar to ideal gas equation,
πV = nRT
Where π = osmotic pressure
R = gas constant
Isotonic solutions
A pair of solutions having same osmotic pressure is called isotonic solution. When two Solutions having the same osmotic pressure are put into communication with each other through a semi permeable membrane, there will be net transference of solvent from one solution to the other, but there is a dynamic equilibrium between two solutions. According to Van’t Hoff equation, it is evident that isotonic solutions must have the same molar concentration, if both are at same temperature.
For isotonic solution, π 1 = π 2
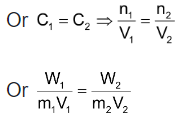
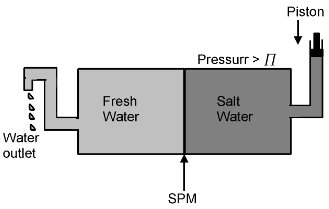
Reverse osmosis and water purification
The direction of osmotic can be reversed if a pressure larger than the osmotic pressure is applied to the solutions side. That is, now the pure solvent flows out of the solution through the semipermeable membrane (SPM). This phenomenon is called reverse osmosis and is of great practical utility. Reverse osmosis is used in the desalination of sea water. A schematic set in the desalination of sea water. A schematic set up for the process is shown in figure. When pressure more than osmotic pressure is applied pure water is squeezed out of the sea water through the membrane. A variety of polymer membrane are available for this purpose.
The pressure required for reverse osmosis are quite high and a workable porous membrane is a film of cellulose acetate placed over a suitable support. Cellulose acetate is permeable to water but impermeable to impurities and ions present in sea water. These days many countries use desalination plants to meet their water requirement.





