
Relative Lowering Of Vapour Pressure
Liquid Solution of Class 12
Relative Lowering Of Vapour Pressure
The addition of a non – volatile solute to a solvent at a given temperature and pressure results in the lowering of the vapour pressure of the solvent.
If a solution is formed by dissolving n moles of a non volatile solute in N moles of a volatile solvent. Then
Mole fraction of solvent, X
1
=
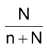
Mole fraction of solute X
2
=
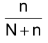
Since the solute is non – volatile, so it would have negligible vapour pressure and thus the vapour pressure of the solution, is inversely the vapour pressure of the solvent.
According to Raoult’s law
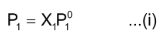
Since X 1 + X 2 = 1 so X 1 = 1 − X 2
Now P
1
=
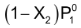
Or
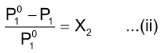
in equation (ii)
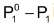 is the vapour pressure lowering and
is the vapour pressure lowering and
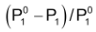 is the relative vapour pressure lowering. From equation (ii) it is clear that the relative vapour pressure lowering is proportional to the mole fraction of the non – volatile solute in solution. It is independent of the nature of the solute. The relative vapour pressure lowering is, therefore a colligative property.
is the relative vapour pressure lowering. From equation (ii) it is clear that the relative vapour pressure lowering is proportional to the mole fraction of the non – volatile solute in solution. It is independent of the nature of the solute. The relative vapour pressure lowering is, therefore a colligative property.
The mole fraction, X
2
=
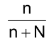
For a dilute solution, the number of moles of the solute (n) can be neglected as compared to the number of moles of the solvent (N). Hence
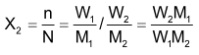
Where W 1 is the amount of the solute dissolved in W 1 amount of solvent, M 1 is the molar mass of the solvent and M 2 is the molar mass of the solute.
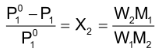
Vapour pressure in terms of molality of the solution:
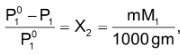 , Where m is the molality
, Where m is the molality









