
Solid Solutions
Liquid Solution of Class 12
Solid Solutions
Solid solutions are formed by mixing two solid components in the molten state in appropriate proportion and then cooling the molten mass. Solid solutions are of two types: substitutional solid solutions and interstitial solid solutions. In a substitutional solid solution, atoms, molecules or ions of one substance take the place of similar species of other substance in its crystal lattice as shown in figure (a). Brass, bronze, monel metal and steel are familiar examples of this type of solid solution.
Interstitial solid solutions constitute the other type and are formed by placing atoms of one kind into voids or interstices, that exist between atoms in the host lattice. This is illustrated in figure (b).
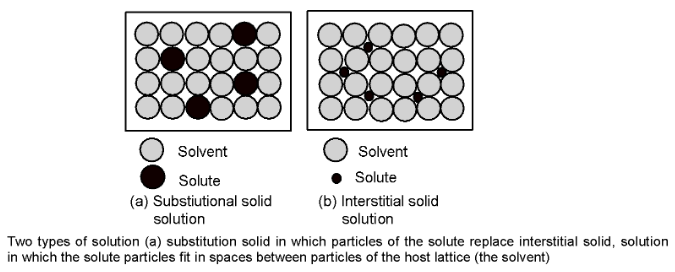
Tungsten carbide. WC. an extremely hard substance is an example of an interstitial solid solution. Here tungsten atoms are arranged in a face-centered cubic pattern with carbon atoms in the octahedral holes where these are surrounded by six tungsten atoms placed at the vertices of the octahedron. Tungsten carbide has many industrial uses in making of cutting and grinding tools.
VAPOUR PRESSURE OF A SOLUTION
Relative lowering of the vapor pressure of a solvent is a colligative property equal to the vapor pressure of the pure solvent minus the vapor pressure of the solution. For example, water at 20°C has a vapour pressure of 17.54 mmHg. Ethylene glycol is a liquid whose vapour pressure at 20°C is relatively low, an aqueous solution containing 0.010 mole fraction of ethylene glycol has a vapour pressure of 17.36 mmHg. Thus the vapour pressure lowering, ΔP = 17.54 mmHg ⎯ 17.36 mmHg = 0.18 mmHg.









