
EVAPORATION
Matter is our surrounding of Class 9
EVAPORATION
The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
Water changes into vapours below 100 o C. The particles of matter are always moving and are never at rest. At a given temperature in any gas, liquid or solid, there are particles with different K.E.
In case of liquids, a small fraction of particles at the surface, having higher K.E., is able to break the forces of attraction of other particles and gets converted into vapour.
FACTORS AFFECTING EVAPORATION:
Surface Area
Greater is the surface area more is the rate of evaporation. This is because only the particles on the surface of the liquid get converted into vapours.
For example, we often spread the wet clothes in air to dry them. By doing so, the surface area available for evaporation of water increases and hence the clothes get dried up soon.
Increase in temperature
The rate of evaporation increases with increase in temperature due to increase in K.E. of liquid particles. This is because, due to increase in K.E., the liquid particles can more easily overcome the forces of attraction of neighbouring particles on the surface of liquid and hence can more easily get converted into vapours.
For example, evaporation of a liquid occurs at a faster rate in summer than in winter.
Decrease in Humidity
By humidity we mean, the amount of water vapour present in air. The air around us can hold only a certain definite amount of water vapours at a particular temperature. Now in case, humidity of air is already high i.e. the amount of water vapours in the air is already high, then air can hold only a little more amount of vapours to reach that optimum level (as air can hold only a certain definite amount of water vapours). Therefore, the rate of evaporation decreases.
For example, we sweat a lot in hot and humid weather than in dry weather because, air already has high amount of water vapours in humid and hot weather. Therefore, the sweat liquid that comes out of our skin does not evaporate and remains sticking to our body.
Increase in the speed of wind
The rate of evaporation increases with increase in wind speed. This is because, due to increase in wind energy, the liquid particles move away with the wind and thus decreasing water vapours in the surroundings.
For example, wet clothes dry faster on a windy day due to increase in wind speed and thereby increasing the rate of evaporation. Similarly, we usually sit under the fan during summer days (when we sweat a lot) because fan increases the wind speed around us, thereby increasing the rate of evaporation and making us feel more comfortable (since evaporation causes cooling).
Nature of Liquid
The rate of evaporation also depends upon the nature of the liquid. In other words, lesser is the boiling point, more is the tendency of the liquid to evaporate or to change into vapours. It can be explained more clearly by the following example :
Alcohol with a boiling point 351K or 78 0 C evaporates much more quickly than water with a boiling point 373K (or 100 0 C). This is because the inter particle force of attraction are weaker in alcohol than in water, so that the particles of alcohol can leave the liquid surface to form vapours more easily than the particles of water and thus rate of evaporation of alcohol is faster than that of water.
|
|
Hence the liquid with less boiling point will evaporate more quickly than the liquid with more boiling point. |
Applications of Evaporative Cooling
1. We sweat so as to cool our bodies. Perspiration is actually evaporation. Water from our body evaporates, taking energy from our body in the process and thus leads to lowering of our body temperature.
2. During the summer, we wear cotton clothes. Cotton, being a good absorber of water permits more sweat to be in contact with the atmosphere, consequently serving to more evaporation. It is for this reason that we feel cooler after we wear cotton clothes.
3. Water is hold in earthen pots to form it cool. The pores of the earthen pot, just like the pores of cotton cloth provide a larger surface area for more evaporation.
The effect of factors like surface area, temperature, humidity and wind speed on the rate of evaporation of liquids can be explained more clearly by performing the following experiment:
Experiment:
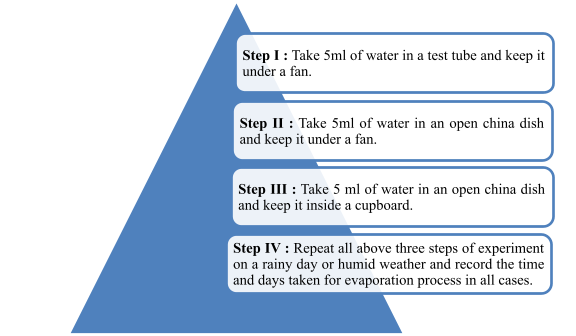
Observation:
- The water taken in a test tube will evaporate slowly than the water taken in two open china dishes.
- The water taken in open china dish placed under fan will evaporate more quickly then water taken in open china dish placed inside a cup-board.
- The first three processes will take longer time for evaporation process on a rainy day or humid weather
Discussion: The surface area of water exposed to atmosphere is minimum in case of test tube, so, it takes a long time (2/3 days) for 5ml of water to evaporate. Although surface area of 5ml of water taken in two open china dishes is the same, yet water in the china dish placed under the fan evaporates more quickly than the water in china dish placed inside a cupboard. This is because wind speed increase due to fan and thereby increases rate of evaporation.
On the other hand, these processes will take longer time for evaporation process on a rainy day or humid weather.
This is due to the reason that
- On a rainy day, temperature is reduced and thus rate of evaporation is decreased
- In a humid weather, the amount of water vapours in air are already high and thus rate evaporation is decreased.
Conclusion: From above discussion we led to conclude that, the rate of evaporation of liquid increases with
- increase in surface area exposed to the atmosphere.
- increase in temperature.
- increase in wind speed.
- decrease in humidity (i.e. amount of water vapours present in air)
Difference between Evaporation and Boiling:
|
S.No. |
Evaporation |
Boiling |
|
1 |
It is a surface phenomenon. |
It is a bulk phenomenon. |
|
2 |
It occurs at all temperatures below B.P. |
It occurs at B.P. only. |
|
3 |
It leaves the cooling effect. |
It increases the temperature. |
Cooling Causes by Evaporation :
The cooling caused by evaporation is based on the fact that when a liquid evaporates, it draws (or takes) the latent heat of vaporisation from ‘anything’ which it touches.
For example :
- If we put a little of petrol on the palm of our hand then our hand feels very cold.
- Perspiration (or sweating) is our body’s method of maintaining a constant temperature.
We Wear Cotton Clothes in Summer :
During summer, we perspire more because of the mechanism of our body which keeps us cool. During evaporation, the particles at the surface of liquid gain energy from the surroundings or body surface. The heat energy equal to latent heat of vaporisation is absorbed from the body leaving the body cool. Cotton, being a good absorber of water helps in absorbing the sweat.
Water droplets on the outer surface of a glass containing ice cold water :
If we take some ice cold water in a tumbler then we will observe water droplets on the outer surface of tumbler.
Reason : The water vapour present in air on coming in contact with cold glass of water, loses energy. So water vapour gets converted to liquid state, which we see as water droplets.









