Formation of Nucleotides
Molecules of Cell of Class 11
Nucleotide Formation
Nucleotides are phosphorylated nucleosides. They are formed by condensation of pentose sugar, a nitrogen base and at least one phosphoric acid residue. Sugar molecule provides attachment to nitrogen base at its carbon 1’ and phosphate at its carbon 5’.
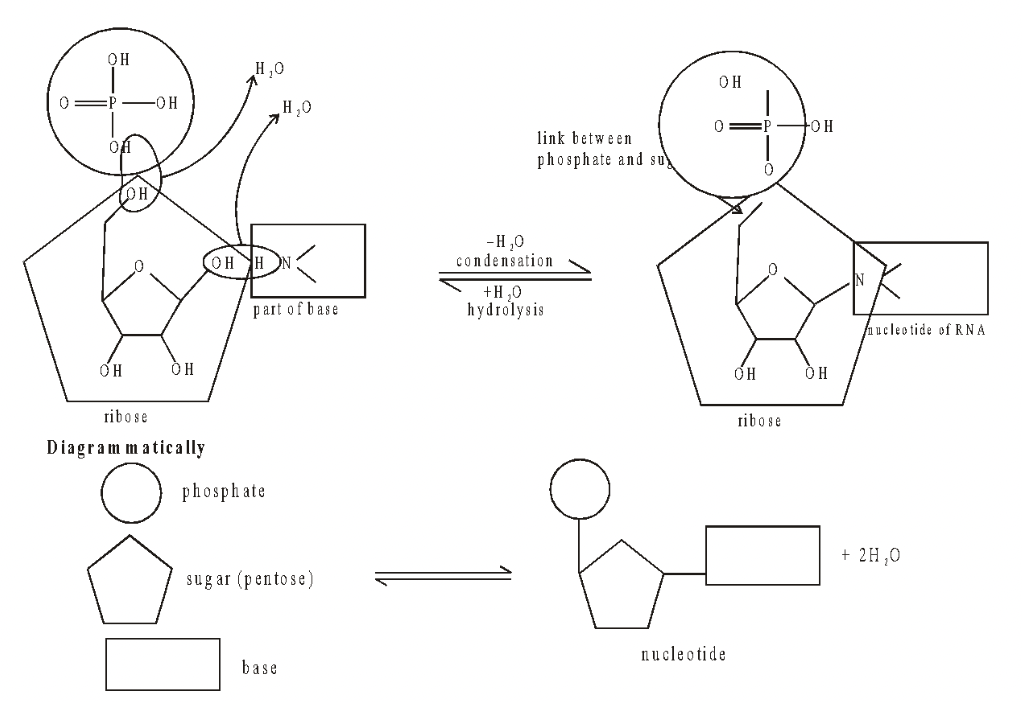
Fig. Formation of a nucleotide
Nucleotides are not only used as building blocks for nucleic acids, but they form several important coenzymes, including adenosine triphosphate (ATP), cyclic AMP, coenzyme A,
nicotinamide adenine dinucleotide (NAD) and its phosphate NADP, and flavine adenine dinucleotide (FAD)
Nitrogen base is connected to pentose sugar by its nitrogen atom 1 in case of pyrimidine (C, T, U) and nitrogen atom 9 in case of purine (A, G).
Various nucleotides are:
Adenosine Monophosphate (AMP) (Adenylic Acid, Adenylate) – Ribose + Adenine + Phosphate
Guanosine Monophosphate (GMP) (Guanylic Acid, Guanylate) – Ribose + Guanine + Phosphate
Cytidine Monophosphate (CMP) (Cytidylic Acid) – Ribose + Cytosine + Phosphate
Uridine Monosphosphate (UMP) (Uridylic Acid, Uridylate) – Ribose + Uracil + Phosphate
Deoxyadenosine Monophosphase (dAMP) (Deoxyadenylic Acid) – Deoxyribose + Adenine + Phosphate
Deoxyguanosine Monophosphate (dGMP) (Deoxyguanylic Acid) – Deoxyribose + Guanine + Phosphate
Deoxycytidine Monophosphate (dCAMP) (Deoxycytidylic Acid) – Deoxyribose + Cytosine + Phosphate
Deoxythymidine Monophosphate (dTMP) (Deoxythymidylic Acid) – Deoxyribose + Thymine + Phosphate
Two nucleotides join to form a dinucleotide by condensation between the phosphate group of one with the sugar of the other. The process is repeated up to several million times to make a polynucleotide.
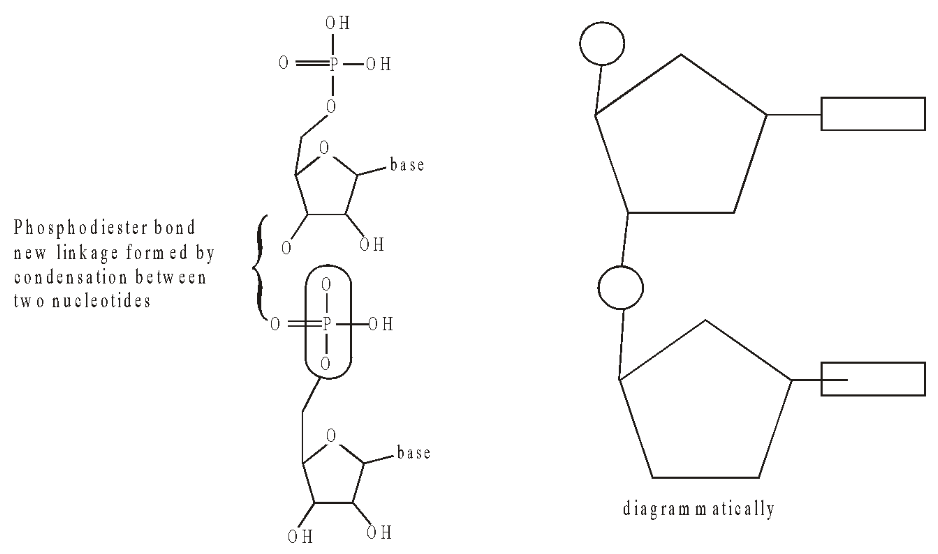
Fig. Structure of a dinucleotide
Cyclic AMP (cAMP)
Discovered by E.W. Sutherland (1956). Cyclic AMP is technically adenosine 3, 5-monophosphate. It is formed from ATP by enzyme adenylate cyclase situated on the inner side of cell membrane. The enzyme is activated by membrane receptor after it comes in contact with suitable hormone or neurotransmitter. The hormone or neurotransmitter is called first messenger
while cAMP functions as second messenger. cAMP triggers a preformed chemical system into activity or inhibition.
Higher Nucleotides
They are nucleotides that possess more than one phosphate radical, e.g. GDP, GTP, CDP, CTP, UDP, UTP, ADP and ATP. All higher nucleotides are ribonucleoside diphosphates and triphosphates.
ATP (Adenosine Triphosphate)
Energy currency or energy depot of the living systems because it is built up from ADP with a small gain of energy and is hydrolysed to release the same amount of energy wherever it is required in the cell.
Chemically it is formed of a purine adenine linked to a pentose sugar ribose which in turn is joined to a row of three phosphate radicals. Starting from ribose, the three phosphate radicals attached to it are designated as
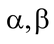 and
and
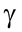 .
.
The α phosphate is attached to ribose by an ordinary chemical bond that carries about 13.8 kJ or 3.3 kcal of energy per mole and β phosphate carries about 27.2 kJ or 6.5 kcal per mole.
The third or γ-phosphate is attached to second or β-phosphate by still higher energy bond with a value of 37.25 kJ or 8.9 kcal per mole.
Discovered by German scientist Karl Lohmann in 1929.
Fritz Lipmann (1941) postulated the concept that ATP is the universal and primary carrier of chemical energy in living cells. Lipmann is also called father of ATP cycle as he was the
first to propose its build up from ADP during exergonic processes and its subsequent breakdown to ADP for providing energy to various cellular activities like biosynthesis, active transport, muscle contraction, etc.
Enzyme taking part in synthesis and hydrolysis of ATP is called ATP-ase.