
Ozone Layer And Its Depletion
Our Environment of Class 10
The global environment is basically formed by three parts – atmosphere, hydrosphere and lithosphere. The atmosphere extends over about 600 km from the earth’s surface. Four layers of atmosphere are – troposphere, stratosphere, mesosphere and thermosphere.
Ozone layer, commonly called ozone blanket, comprises of high concentration of ozone about 18-26 km above in the stratosphere. As per estimates, 90% of the total atmospheric ozone is present in this region.
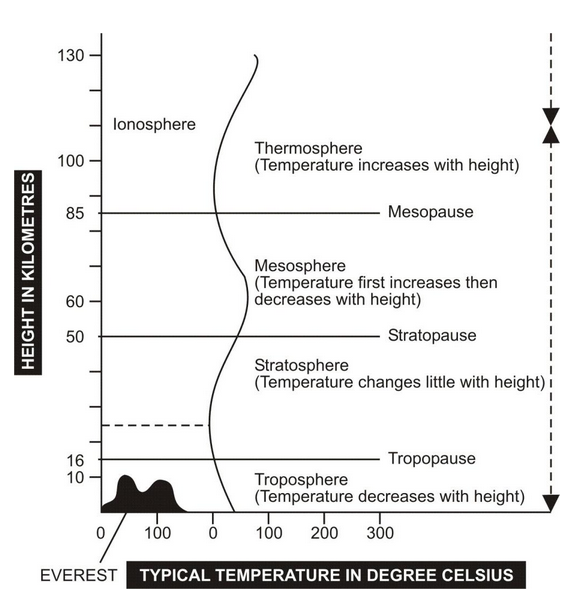
Main layers of the atmosphere. These are shown between solid lines. The vertical wavy line depicts temperature variation with increasing height.
Formation of ozone:
Ozone is a form of oxygen. Ozone (O 3 ) is a molecule formed by three atoms of oxygen. While O 2 , which we normally refer to as oxygen, is essential for all aerobic forms of life. Ozone at the higher levels of the atmosphere is a product of UV radiation acting on oxygen (O 2 ) molecule. The higher energy UV radiations split apart some molecular oxygen (O 2 ) into free oxygen (O) atoms. These atoms then combine with the molecular oxygen to form ozone.
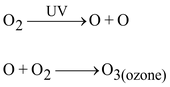
Function of ozone:
Ozone is a poisonous gas but is not stable nearer to earth’s surface. It performs an essential function where it is found. It absorbs the harmful radiations from the Sun. It shields the surface of the earth from the ultraviolet (UV) radiation of the sun. Absorption of UV radiations by ozone blanket is proportional to its thickness.
OZONE LAYER DEPLETION:
Between 20 and 26 km above the sea level ozone layer is present and the part of atmosphere containing it is called ozonosphere (Stratosphere). This layer is established due to an equilibrium between photo dissociation of ozone by UV - radiations and regeneration of ozone. The thickness of this ozonosphere averages 5 km. The ozone layer acts as an ozone shield and absorbs the harmful UV-radiations of the sunlight so protects the earth's biota from the harmful effects of strong UV-radiations. So this layer is very important for the survival and existence of life on earth.
(a) Causes of Thinning of Ozone Layer: The decline in spring - layer thickness is called ozone hole. Ozone hole is largest over Antarctica and was just short of 27 million sq. km. during September 2003. Main chemicals to be responsible for destruction of ozone-layer are: chlorofluorocarbons (CFCs), halogens (used in fire extinguishers), methane and nitrous oxide. Out of these, most damaging is the effect of CFCs which are a group of synthetic chemicals and are used as coolants in refrigerators and air conditioners; as cleaning solvents, propellants and sterilant etc. These CFCs produce "active chlorine" in the presence of UV-radiations. These active chlorine radicals catalytically destroy ozone and convert it into oxygen. Ozone at the higher levels of the atmosphere is a product of UV radiation acting on oxygen (O2) molecule. The higher energy UV radiations split apart some molecular oxygen (O2) into free oxygen (O) atoms. These atoms then combine with the molecular oxygen to form ozone as shown –

In 1987, the United Nations Environment Programme (UNEP) succeeded in forging an agreement to freeze CFC production at 1986 levels.
- Nitrous oxide: is produced in industrial processes, forest fires, solid waste disposal, spraying of insecticides and pesticides, etc. Methane and nitrous oxide also cause ozone destruction.
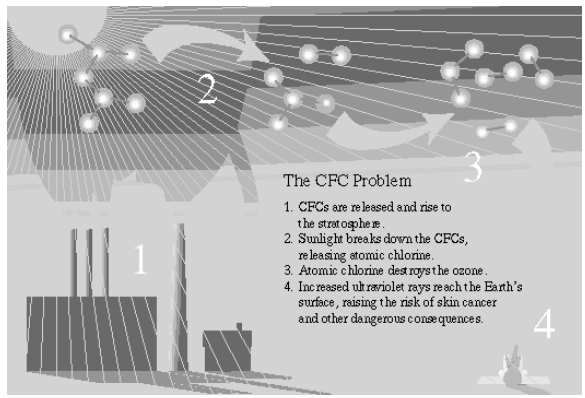
Depletion of ozone layer
Effects of Ozone Layer Depletion : The thinning of ozone layer results in increase in the UV radiations (in the range of 290 - 320 nm) reaching the earth's surface. It is estimated that 5 percent loss of ozone results in 10 per cent increase in UV - radiations. These UV - radiations can:
(i) Increase in incidences of cataract and skin cancer.
(ii) Decrease in the functioning of immune system.
(iii) Inhibit photosynthesis in most of phytoptanktons so adversely affecting the food chains of aquatic ecosystems.
(iv) Damage nucleic acids of the living organisms.
Saving the ozone layer:
The thinning of ozone layer started in 1980s. In 1987, the United Nations Environment Programme (UNEP) succeeded in forging an agreement to freeze CFC production at 1986 levels.










