
Atomic Mass And Molecular Mass
Some Basic Concept Of Chemistry of Class 11
Atomic Mass And Molecular Mass
Atomic Mass: As atoms are very tiny particles, their absolute masses are difficult to measure. However it is possible to determine the relative masses of different atoms if small unit of mass is taken as standard (previously, this standard was mass of one atom of hydrogen and taken as unity. Later on it was 1/16 th part of oxygen atom and now it is 1/12 th part of C−12 atom).
The atomic mass of an element can be defined as the number which indicates how many times the mass of one atom of the element is heavier in comparison to the mass of one atom of hydrogen.
Atomic mass of an element
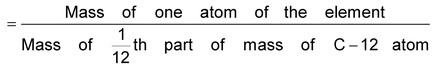

Atomic Mass Unit:
The quantity 1/12 mass of an atom of carbon−12 is known as the atomic mass unit and is abbreviated as amu. The actual mass of one atom of carbon−12 is
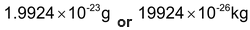
Thus 1 amu
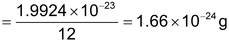
Atomic mass of an element
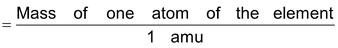
Actual mass of an element

Determination of atomic mass
(i) Applying Dulong and Petit’s law.
(ii) Cannizzaro’s methods
(iii) By mitscherlich’s law of isomorphism.
(iv) By measurement of V.D. of volatile chloride or bromide.
(i) Dulong & Petits Law: The product of specific heat of pure element and atomic mass of the element is equal to 6.4.
i.e. Atomic mass × specific heat = 6.4 (approx)
But this law is not applicable to lighter element like boron, carbon, silicon. To obtain correct atomic mass of element first of all equivalent mass of the element is known by any other method and their atomic mass = eq. weight × valency
In which valence has whole number value which can be deduced by dividing approximate by equivalent mass.
Dulongs and Petit’s Law:

(ii) Cannizzaro’s methods
If an element has several compound with other same or different elements of known atomic mass then the compound that has minimum presence of former element indicate the atomic mass of former element.
Procedure
(i) First of all the molecular mass of all compound known by applying
V.D × 2 = mol. weight
(ii) By analysis the presence of the desired element in each compound is known.
(iii) The mass that is lowest among al their compound indicate the atomic mass of their element.
(iii) Law of Isomorphism
When two or more compound forms similar type of crystals or able to form mixed crystals. They are known as isomorphs. For examples: MgSO4.7H2O, ZnSO4.7H2O and FeSO4.7H2O are isomorphs of each other as their crystals posses same shape.
According to mitscherlich [year 1819].
The valency of elements that are similarity placed to that of other elements in their isomorphs are always same.
In the above example Fe, Zn and Mg have same valency [2] and equal ratio of water molecule in each isomorphs.
If equivalent mass of one element is known then atomic mass can be calculated by knowing the valency of other isomorphs key element.
(iv) Atomic mass from vapour density of a chloride:
The following steps are involved in this method
- Vapour density of chloride of the element is determined
- Equivalent mass of the element is determined
Let the valency of the element be x. The formula of its chloride will be MClx
Molecular = Atomic mass of M + 35.5 x
Atomic mass = x x E
So Mw = E x x + 35.5x = 2V>..
x - 2VD/E+35.5
AVERAGE ATOMIC MASS
Elements are found in different isotopic forms (atoms of same elements having different atomic mass), so the atomic mass of any element is the average of all the isotopic mass within a given sample.
Average atomic mass
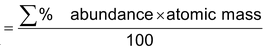
GRAM ATOMIC MASS OR GRAM ATOM
Atomic weight of an element in grams is called as Gram atomic mass of an element. Gram atomic mass is the weight of 1 mole atom (6.023 x 10 23 atoms ) of the element. It is also called as 1 gram atom. e.g. AW of C = 12, GAW of
C = 12g = 6.023 x 10
23
atom of carbon = 1 mole atom of
C = 1 gram atom of carbon.
Molecular Mass
Number of times a molecule of a compound is heavier than one atom of hydrogen or 1/12 th part of C−12 is molecular mass of the compound. It is the sum of the atomic mass of atoms present in a molecule.
For example Molecular mass of CO 2 = 44
i.e 1 molecule of CO 2 is 44 times heavier than one atom of hydrogen or 1/12 th part of C −12.
Gram Molecular Mass
Molecular mass expressed in grams is called as gram molecular mass. It is the weight of one mole molecules of a compound. It is also called as one gram molecule.
For example GMW of CO 2 = 44g = 1 mole molecule of CO 2 = 6.023 x 10 23 molecules
= 1 gram molecule of CO 2









