
Charles Law Formula has vital applications in the domain of chemistry. Jacques Charles, a French scientist, researched how temperature affects a gas's volume at constant pressure. He lived from 1746 to 1823. Charles' Law states that, when pressure is kept constant, a given amount of gas will change in volume in direct proportion to its absolute temperature. The absolute temperature is calculated using the Kelvin scale, which is used to measure temperature. It must be used because 0 on the Kelvin scale signifies a complete cessation of molecular motion.
Introduction to Charles Law Formula
Charles' law states that the volume of an ideal gas at constant pressure is inversely proportionate to its absolute temperature. According to the law, the Kelvin temperature and volume will be directly related when the pressure on a sample of a dry gas is kept constant. The following mathematical formula is used to represent the Charles Law: V/ T =k, Where V represents the volume of the gas, T represents the temperature of the gas and k represents the Charles constant. In other words, Charle's law is a modification of the ideal gas law. The law holds true for ideal gases that are kept at constant pressure, but the temperature and volume vary at all times.Charle's Law Formula
The formula for Charles law is represented as V i/ T i = V f/ T f In this, V i =Initial volume, T i =Final volume, V i =Initial volume, V f =Final volume. The value of temperature should only be taken in Kevin values and not in celsius.Download PDF Charle's Law Formula
Derivation of Charles Law Formula
As per the definition of Charles Law, under constant pressure, the volume of the gas remains directly proportional to the Kelvin temperature. This implies that if there is an increase in the volume, the temperature of the gas will also rise proportionally. This can be expressed as V∝T This relation can be mathematically equated as V/ T =k So, if the initial volume of the gas is V i and the initial temperature of the gas is T i , Then the equation can be written as V i/ T i =k (1) Now, assuming the final volume of the gas to be V f and the final temperature of the gas to be T f , the equation becomes V f/ T f =k (2) Combining (1) and (2) we can obtain, V i/ T i = V f/ T f or V i T f= V f T i This is the final Charles Law Formula. In order to answer problems with Charles law, it is crucial to understand that the unit of temperature must be in Kelvin rather than Celcius or Fahrenheit, as was already mentioned above. The absolute temperature scale also refers to the temperature measured in Kelvin. The temperature in Celsius must be multiplied by 273 in order to be converted to Kelvin. A change in temperature of one Kelvin unit is equivalent to a change in temperature of one Celsius degree. Always keep in mind that the Kelvin scale's zero represents -273, or "Absolute Zero." When the gas is at a fixed mass and pressure, the density of the gas is inversely proportional to the temperature in Kelvin.Graphical Representation of Charles Law Formula
The Charles Law Formula can be graphically represented as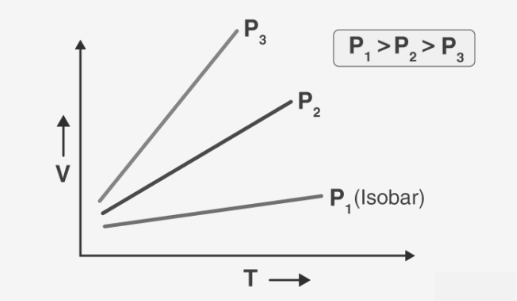
Applications of Charles Law Formula
(1) Hot Air Balloon
An envelope that holds heated air, a burner, and a basket or gondola for passengers make up the majority of a hot air balloon's parts. The air inside the envelope warms up when the fuel source is lit. According to Charles' Law, the volume of the air will grow as the air's temperature rises. Therefore, as a result, the air density inside an enclosure becomes lower than the air density outside. Therefore, a hot air balloon floats high in the sky due to buoyant force.(2) Baking
When bread and cakes are fermented, yeast is employed to create a soft, pliable texture. Carbon dioxide gas is generated by yeast. When bread and cakes are baked at high temperatures, carbon dioxide gas expands as the temperature rises. This expansion makes our bread and cakes seem pleasantly spongy and fluffy and makes them ready to be served.(3) Soda Can
Charles' law states that a chilled soda can's volume decreases as a result of the low temperature. That explains why the Coke can barely have any bubbles coming out of it. On the other hand, a warmer soda can has more volume because of the higher temperature. That explains why the drink is leaking bubbles.Charles Law Formula FAQs
What are Charles Law's restrictions?
Only ideal gases fall under the scope of Charles' law. Charles's law is only applicable to actual gases at low pressures and high temperatures. At high pressures, there is a nonlinear relationship between the quantity and temperature.
What practical application does Charles Law have?
Charles Law states that a cooler temperature reduces the number of gases in a can of chilled soda. There are fewer bubbles inside it as a result. While in a warmer soda, a greater temperature causes an increase in volume, which causes the drink to pour out.
Where is Charles Law relevant?
The application of Charles' law also indicates that the relationship between a gas's density and absolute temperature is inverse as mass and pressure rise. Observations of the weather and the use of air balloons in sports are two examples of real-world uses of Charles' law.
Is there an inverse association in Charles Law?
Charles's Law is an inverse relationship, in contrast to Boyle's Law, which is a direct relationship. Although pressure and temperature are included in each rule, both deal with volume.
Why does Charles Law assume constant pressure?
The weight will apply a continuous downward force to the piston if both objects remain still. This force is countered by an equivalent upward force brought about by the gas pressing up on the piston's inner surface.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









