
The chemical effect of electric refers to the phenomenon where an electric current passing through a conducting medium causes chemical reactions to occur. This effect is used in various applications and technologies, including electroplating, electrolysis, and the operation of batteries.
What are the Chemical Effects of Electric?
When an electric current travels through a substance, changes in the chemical makeup or characteristics of that substance take place. Electric charges and the atoms or molecules of a substance interact to produce these effects.Also Read - Human Eye and Colourful World Formula
Important Formulas of Chemical Effects of Electric
The chemical effect of electric refers to the phenomenon where an electric current passing through an electrolyte causes a chemical reaction to occur, resulting in the deposition of substances on electrodes or the dissolution of electrodes. This is the basis of processes like electroplating and electrolysis. The key formula related to the chemical effect of electricity is Faraday's Laws of Electrolysis.- Faraday's First Law of Electrolysis
- m is the mass of the substance deposited or liberated.
- z is the electrochemical equivalent of the substance (grams per coulomb).
- F is Faraday's constant, which is the charge of one mole of electrons (approximately 96,485 C/mol).
- Q is the electric charge passed through the electrolyte in coulombs.
- Faraday's Second Law of Electrolysis
- m 1 , m 2 , m 3 … are the masses of substances deposited or liberated.
- z 1 , z 2 , z 3 … are the electrochemical equivalents of the substances.
- Conduction
- Insulation
- Semiconductors
- Resistance
Also Read - Light Formula
Applications of Chemical Effects of Electric
The chemical effects of electric have a wide range of practical applications in various fields. Here are some notable applications:- Electroplating
- Electrorefining
- Electrolysis
- Battery Charging and Discharging
- Electrochemical Sensors
- Electrochemical Machining
- Electrolytic Cells in Industry
- Electrochemical Analysis
- Water Treatment
- Electrochemical Etching
Also Read - Source of Energy Formula
Electric Current in a Conductor
Electric current in a conductor refers to the flow of electric charge through the conductor. It is the movement of electrons or other charge carriers in response to an applied electric field. Formula I=nAeV. v d =λ/τ, v d =1/2(eE/m)τ 2 =1/2 eE/m τ, I=neAV dElectrical Resistance
Electrical resistance is a fundamental property of materials that quantifies how strongly they oppose the flow of electric current. It is a crucial concept in understanding and analysing electrical circuits and the behaviour of materials in response to applied electric fields. I=neA V d =neA( eE/ 2m) τ =( n e 2 τ/ 2m) AE E= V/ l so I=( n e 2 τ/ 2m)( A/ l) V=( A/ρ l) V=V/R ⇒ V=IR- ρ is called the resistivity
- ρ= 2m/ n e 2 τ = 1/σ
- ρ is called conductivity
Current Density
Current density ( J ) is a fundamental concept in electromagnetism that quantifies the amount of electric current flowing through a unit cross-sectional area of a conductor. It provides valuable information about the distribution of current within a conductor and is often used in the analysis and design of electrical systems.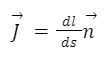 Here are the key points to understand about current density:
Here are the key points to understand about current density:
- Direction of Current Density
- Relation to Current
- Heterogeneous Materials
- Relation to Ohm's Law
- Applications
- Temperature Effects
Electrical Power
Electrical power refers to the rate at which electrical energy is consumed, produced, or transferred in an electrical circuit or device. It is a fundamental concept in understanding the performance, efficiency, and operation of various electrical systems. P=VI Energy =∫ pdt P= I 2 R=VI= V 2 / R H=VIt= I 2 Rt= V 2 / R t H= I 2 RT Joule= I 2 RT/ 4.2 Calorie Understanding electrical power is essential for evaluating the performance, efficiency, and safety of electrical systems, as well as for designing and optimising various electrical devices and applications.Chemical Effect of Electric Formula FAQs
What are the chemical effects of electric?
The chemical effects of electric refer to the phenomenon where an electric current passing through an electrolyte (a substance that conducts electricity when dissolved in water or melted) causes chemical reactions to occur, leading to the deposition of substances on electrodes or the dissolution of electrodes.
What are some examples of chemical effects of electricity?
Examples of chemical effects of electricity include electroplating (depositing a metal layer on an object), electrolysis (using electric current to induce chemical reactions), and electrochemical reactions in batteries and fuel cells.
How does electroplating work?
Electroplating involves passing an electric current through an electrolyte containing metal ions. The metal ions are reduced at the cathode (the object to be plated), forming a metal layer on its surface. For example, in copper electroplating, a copper object is made the cathode, and a copper sulphate electrolyte is used.
What is electrolysis?
Electrolysis is a process where an electric current is used to drive a nonspontaneous chemical reaction. It involves the decomposition of compounds into their constituent elements or ions. For instance, electrolysis of water breaks down water molecules into hydrogen and oxygen gases.
How is electrolysis used in industry?
Electrolysis has various industrial applications, including the production of chlorine gas and sodium hydroxide through the chlor-alkali process, extraction of metals from their ores, and electroplating for surface finishing and corrosion protection.
What is Faraday's law of electrolysis?
Faraday's laws of electrolysis describe the quantitative relationships between the amount of substance deposited or liberated during electrolysis and the amount of electric charge passed through the electrolyte. The laws provide a foundation for understanding the chemical effects of electric.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.









