
Displacement Reactions (Single & Double Displacement)
Nov 02, 2022, 16:45 IST
Displacement reactions is very important chemical reactions of chemistry. They are used in various ways in various fields. For example, we use electroplating to prevent iron objects from rusting, which is based on displacement reaction.
What is a Displacement Reaction?
A displacement reaction is where another atom in a molecule displaces the atom or a set of atoms. For example, it replaces the copper metal when an iron is added to a copper sulphate solution.
A + B - C → A - C + B
The given above equation exists when A is more reactive than B.
A and B have to be either:
- Halogens where C indicates a cation.
- Different metals wherein C indicates an anion.
There are two types of displacement reactions:
- Single displacement reaction
- Double displacement reaction
Single Displacement Reaction
A single displacement reaction, also known as single replacement reaction, is that type of oxidation-reduction chemical reaction where an ion or element is displaced from a compound, i.e., one element is replaced by another.
When chlorine (or as a gas dissolved in water) is added to a sodium bromide solution, chlorine is obtained instead of bromine. Since the chlorine is more reactive than the bromine, it displaces bromine from the sodium bromide, and the solutions turn blue. The brown color is extruded bromine. If you notice the equation, you can see that Cl and Br have swapped their original places.
Cl 2 (aq) + 2NaBr(aq) → 2NaCl(aq) + Br 2 (aq)
Types of Chemical Reaction
- Synthesis Reactions
Synthesis reactions happen when the two separate atoms or molecules come together to generate a new molecule or substance.
E.g., Water:
2H 2 (g)+O 2 (g)→2H 2 O(g)
- Decomposition Reactions
There are three main types of breakdown reactions:
- Reaction of Thermal Decomposition:
A decomposition reaction that is activated by thermal energy is known as a thermal decomposition reaction.
E.g., CaCO 3 →CaO+CO 2
Calcium carbonate decomposes into carbon dioxide and calcium oxide when heated. This procedure is used to form quick lime, which is a critical ingredient in a variety of industries.
- Reaction of Electrolytic Decomposition:
An activation energy for decomposition in electrolytic decomposition reaction is transferred in the form of electrical energy. The electrolysis of water is the example of an electrolytic breakdown reaction, which may be represented by the chemical equation:
E.g., 2H 2 O→2H 2 +O 2
Examples of Single Displacement Reaction
- The reaction between hydrochloric acid and zinc:
2HCl(aq)+Zn(s)→ZnCl 2 (aq)+H 2 (g)
- The reaction between sodium chloride and fluorine:
2NaCl(aq)+F 2 (g)→2NaF(s)+Cl 2 (g)
- The reaction between calcium iodide and chlorine:
CaI 2 (s)+Cl 2 (g)→CaCl 2 (s)+l 2 (s)
- The reaction between calcium and fluoride, and bromine:
CaF 2 (s)+Br 2 (ℓ)→CaBr 2 (s)+F 2 (g)
Double Displacement Reaction
Double displacement reactions usually occur when a part of two ionic compounds is exchanged and makes the two new components. Below is the pattern of a double displacement reaction is like this.
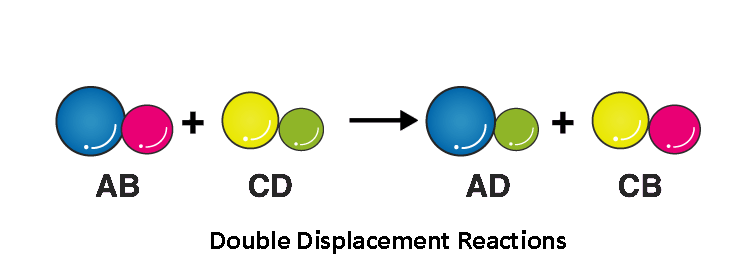
Double displacement reactions mostly take place in aqueous solutions, where ions are precipitated, and ion exchange occurs.
For example, when the solution of an barium chloride is mixed with sodium sulphate, a white precipitate of barium sulphate quickly forms. These reactions are ionic. Reactants are converted into ions when dissolved in water, and ion exchange occurs in the solution, forming a product molecule.
Examples of Double Displacement Reaction
- The reaction between silver nitrate and sodium chloride:
AgNO 3 + NaCl → AgCl + NaNO 3
- The reaction between sodium chloride and calcium sulphate:
2NaCl + CaSO 4 →Na 2 SO 4 + CaCl
- The reaction between sulphuric acid and lithium hydroxide:
H 2 SO 4 + 2LiOH ⇄ LiO 2 SO 4 + 2H 2 O
- The reaction between silver nitrate and hydrochloric acid:
AgNO 3 + HCl ⇄ AgCl + HNO 3
- The reaction between lead(II) nitrate and sodium chloride:
Pb(NO 3 ) 2 + 2NaCl ⇄ 2NaNO 3 + PbCl
- The reaction between the hydrochloric acid and the sodium hydroxide:
HCl + NaOH → NaCl + H 2 O
Uses of Displacement Reaction
- Welding using Thermite
The railway joints are welded together using an alloy of aluminium (Al) and ferric oxide (Fe2O3).
2Al + Fe 2 O 3 → Al 2 O 3 + 2Fe
- Iron Ore Extraction
By reacting with the carbon, iron may be removed from its ore.
3C + 2Fe 2 O 3 → 4Fe + 3CO 2
- Metals are Extracted
The other metals, such as chromium, can be extracted via a displacement reaction.
3C+ 2Cr 2 O 3 → 4Cr + 3CO 2
- Neutralisation of Acids
The indigestion is caused by the production of HCl acid in our stomach. An antacids are made up of a base that causes a displacement response in the body.
Mg(OH) 2 + 2HCl → MgCl 2 + 2H 2 O
Applications of Displacement Reactions
- It is used in thermite welding where aluminium displaces iron from its oxide.
- It is used in steel making where carbon displaces iron from its oxide.
- It is widely used in the extraction of metals.
- It is used in acid indigestion.
- It is used in flame photometry.
Frequently Asked Question (FAQs)
Q1. How will you know if a reaction is a displacement?
Ans. Elements can be placed into a reactivity series, with the most reactive element at the top list and the least reactive at the bottom list. A displacement reaction occurs when a more reactive element displaces or pushes out a less reactive element from a compound that includes the less reactive element.
Q2. Give one example of displacement reaction
Ans. When a iron is added to a copper sulphate solution, it displaces the copper metal.
Q3. What are the 2 types of displacement reaction?
Ans. There are two types of double displacement reactions: precipitation and neutralization.
- Precipitation reaction: In a precipitation reaction, the ions of the reactants compound interchange to form new compounds.
- Neutralization reaction: Neutralization reactions occur between acids and bases and form a salt and a gas.
Q4. Which reactions are single displacement reactions?
Ans. A single replacement reaction, sometimes called a single displacement reaction, is a reaction in which 1 element is substituted for the another element in a compound. The starting materials are always pure elements, such as a pure zinc metal or hydrogen gas, plus an aqueous compound.
Q5. Is rust a single displacement reaction?
Ans. A real-world example of a single replacement reaction is the formation of rust.
Q6. Is Neutralisation a displacement reaction?
Ans. A neutralization reaction is a double displacement reaction becuase it produce salt and water between an acid and a base. The salt is formed from both the negative ion of the acid and the positive ion of the base.
Q7. Is rusting of iron a displacement reaction?
Ans. It can generally be seen as rusting involving oxidation. It may seem like a displacement reaction, but it is an oxidation reaction in which the oxidation number of the iron increases from 0 to 3 as Fe to Fe 3+ .









