
Formula For Salts of Strong Acids and Weak Bases
Sep 06, 2022, 16:45 IST
Salts of strong acids and weak bases [SA-SB]
Such salts give acidic solutions in water. Some of such salts are : NH 4 Cl, ZnCl 2 , FeCl 3 etc. For the purpose of discussion, we will consider hydrolysis of NH 4 Cl. For More C hemistry Formulas just check out main pahe of Chemsitry Formulas.
When NH 4 Cl is put in water, it completely ionises in water to give NH 4 + and Cl - ions. NH 4 + ions combine with OH - ions furnished by weakly dissociated water to form NH 4 OH (weak base). Now for keeping K w constant, water further ionises to give H + and OH - ions, where OH - ions are consumed by NH 4 + ions leaving behind H + ions in solution to give an acidic solution.
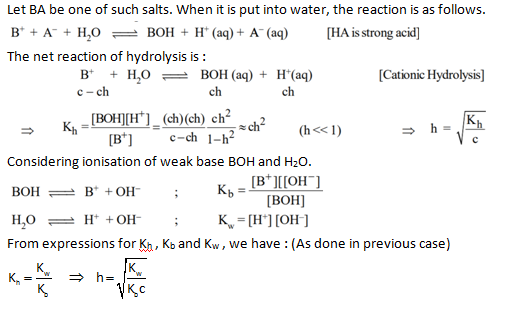
pH of solution :
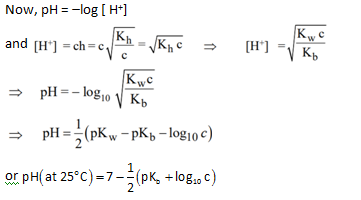
Example For Salts of Strong Acids and Weak Bases
Example 1
The basic ionisation constant for hydrazine, N 2 H 4 is 9.6× 10 –7 . What would be the percent hydrolysis of 0.1 N 2 H 5 Cl ?
A. 0.016
B. 3.2
C. 1.6
D. 0.032
Ans. D
Example 2
The pH of a 0.02 M aqueous solution of NH 4 Cl is equal to
A. 3.78
B. 4.73
C. 5.48
D. 7.00
Ans. A









