
It is a halogen and its most commonly known compound is sodium chloride (NaCl). It is widely used in drinking water as a bleaching agent to eliminate viruses and bacteria. Its other uses include dyeing, pharmaceuticals, automotive, and agriculture.
Chlorine Gas Formula Structure
The chemical element Chlorine Gas Formula has the symbol Cl and atomic number 17. Among the halogens, it is the second lightest. In the periodic table, Chlorine Gas Formula is located between fluorine and bromine, with most of its properties intermediate between them. The colour of chlorine at room temperature is yellow-green. Based on the revised Pauling scale, it has the highest electron affinity and the third-highest electronegativity among the elements, behind only oxygen and fluorine. In addition to the revised Pauling scale, nitrogen’s electronegativity is also listed as greater than chlorine’s on several other scales, including Allen, Allred-Rochow, Martynov-Batsanov, Mulliken-Jaffe, Nagle, and Noorizadeh-Shakerzadeh. Medieval alchemists commonly heated chloride salts like ammonium chloride and sodium chloride as part of their experiments. As a result, hydrogen chloride, mercury (II) chloride (corrosive sublimate), and hydrochloric acid (as aqua regia) are produced. Chlorine Gas Formula was first recognized as a separate substance around 1630 by Jan Baptist van Helmont, and Carl Wilhelm Scheele described it as an oxide of a new element in 1774.
Properties of Chlorine Gas Formula
| Cl 2 | Chlorine gas |
| Density | 3.2 g/L |
| Molecular Weight/ Molar Mass | 70.90 u |
| Boiling Point | -34.04 °C |
| Melting Point | -101.5 °C |
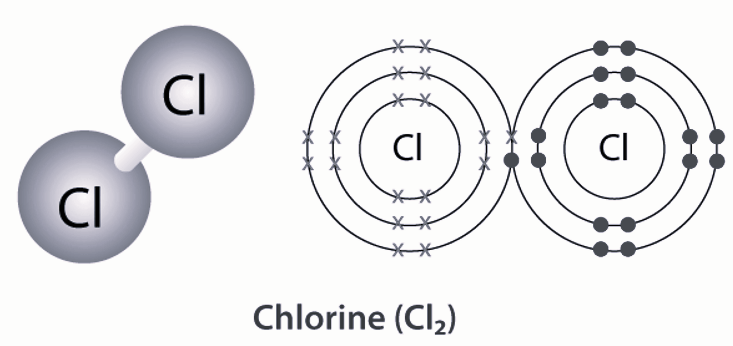
| Chemical Formula | Cl 2 |
The chemical formula for chlorine gas is Cl 2 , which is yellow-green. It also has a similar smell to household bleach. When mixed with water, it forms hypochlorous and hydrochloric acids. High chlorine gas levels can irritate the skin, resulting in tissue damage, burning, blistering, and tingling sensations. In addition, when it gets in contact with the eyes, it can cause severe burns. It is important to note that chlorine gas is highly poisonous and can lead to loss of consciousness if inhaled at high concentrations. Its molecular weight is 70.906 grams and liquid density is 3.2 grams per milliliter. Lastly, its freezing point is -101.5 degrees Celsius and boiling point is -34.038 degrees Celsius.
Physical Properties of Chlorine Gas – Cl 2
| Odour | Odour of bleach |
| Appearance | Yellow-green gas |
| Covalently-Bonded Unit | 1 |
| Vapour Pressure | 85.3 psig |
| pH | 7.4 |
| Solubility | Slightly soluble in water |
Also Check – Molecular Speed Formula
Chemical Properties of Chlorine Gas – Cl 2
- As a result of chlorine's reaction with organic compounds and ammonia, chloro-organics and chloramines are formed.
- Chloramines are part of a class of chlorine compounds known as disinfectants, which appear in chlorine residue tests. As a reducing agent in wastewater, it is known as chlorine demand. The reaction is as follows. H 2 O + Cl 2 → HCl + HClO
Uses of Chlorine Gas – Cl 2
- In the First World War, the Germans used chlorine gas as a chemical weapon against allied forces.
- Disinfection is the most common use of chlorine in wastewater treatment.
- In addition to controlling odours and controlling filamentous organisms in activated sludge, it is used to prevent the spread of waterborne diseases, and is the most commonly used disinfectant.
Also Check – Ionization Energy Formula
Health Hazards
Concentrated vinegar is highly dangerous and can be fatal when breathed in. It can irritate the eyes and skin, and due to its strong oxidizing properties, there is a risk of fire. Aquatic life is also harmed by it. Similarly, exposure to high levels of chlorine gas can result in severe irritation and tissue damage on the skin, causing symptoms such as burns, blisters, and tingling sensations. The gas can also cause serious burns if it gets into the eyes. Inhaling large quantities of this poisonous gas can lead to unconsciousness.
Also Check – Internal Energy Formula
Chlorine Blood Test
A chlorine blood test can diagnose illnesses caused by chlorine in any form and provide information on the amount of chlorine present in our bodies. As one of the important electrolytes in our body, chlorine plays a crucial role in regulating body fluids, acid levels, and other physiological activities. Checking the levels of chlorine can also help detect kidney disease, liver failure, heart disease, and changes in blood pressure. In case of an acid or fluid imbalance, a chlorine blood test is recommended. This test not only measures the chlorine content but also provides a profile of other electrolytes. Sometimes, fasting for several hours may be required before taking this test upon doctor's advice. Abnormal levels of chlorine could indicate dehydration, kidney failure, liver diseases, or imbalances like acidosis and alkalosis. On the other hand, low levels of chlorine may suggest heart failure, lung disease or Addison’s disease - a condition where the adrenal glands do not secrete enough hormones causing symptoms such as dizziness, weight loss and dehydration.
Chlorine Gas Formula FAQs
Q1. What is the chemical formula of chlorine gas?
Q2. Is chlorine gas naturally occurring, or is it typically produced?
Q3. What are the main uses of chlorine gas?
Q4. Is chlorine gas toxic to humans?
Q5. How does chlorine gas contribute to the greenish color of swimming pools?










