
Gas Pressure Formula: Pressure is defined as the force exerted by a substance on another substance per unit area. In the context of gases, gas pressure specifically refers to the force exerted by gas molecules on the boundaries of their container. These gas molecules exhibit random motion within a given volume, resulting in frequent collisions with both the container's surface and each other. Although the impact of an individual gas molecule is minuscule and challenging to observe, the cumulative effect of all gas molecules collectively constitutes the overall gas pressure. It follows that the more collisions occurring, the higher the pressure will be.
The formula for gas pressure can be expressed as follows:
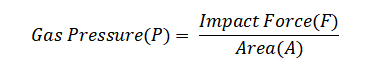
Where:
F represents the impact force due to gas collisions in Newtons (N), and
A denotes the area in square metres (m²).
Additionally, an ideal gas pressure formula is applicable, which relates pressure to other gas properties:
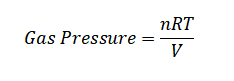
Where:
n is the number of moles,
R is the gas constant (8.3145 J/mol/K),
T is the temperature in Kelvin (K), and
V stands for volume.
The standard unit for measuring gas pressure in the International System of Units (SI) is the Pascal (Pa).
Also Check - Theoretical Yield Formula
Gas Pressure Formula Solved Example
Example 1: How do you determine the gas pressure when you have 500 moles of gas molecules in a container with a volume of 40 L, and the temperature is 220 K?
P= nRT / V
Substituting the given values:
n=500 mol,
T=220 K,
V=40 L.
Thus, the gas pressure (P) is calculated as:
P= 500×8.3145×220 / 40 = 225.5 Pascals (Pa)
P=225.5Pascals (Pa)
Also Check - Vapor Pressure Formula
Example 2: How can you calculate gas pressure when gas molecules apply a force of 300 N on an area of 50 m², using the basic pressure formula?
P= F / A
Substituting the provided values:
F=300 N,
A=50 m².
Thus, the gas pressure ( P) is calculated as:
P = 300 / 50 = 6 Pascals (Pa)
P= 6 Pascals (Pa)
Also Check - Dilution Formula
Example 3: Calculate the gas pressure when you have 2 moles of gas in a container with a volume of 10 liters at a temperature of 300 Kelvin.
Using the ideal gas pressure formula:
P = nRT / V
Substituting the given values:
n = 2 mol,
T = 300 K,
V = 10 L.
P = (2 × 8.3145 × 300) / 10 = 498.87 Pascals (Pa)
P = 498.87 Pascals (Pa)
Example 4: Determine the gas pressure when you have a container with 0.5 moles of gas at 25 degrees Celsius (298 Kelvin) and a volume of 2 liters.
Using the ideal gas pressure formula:
P = nRT / V
Substituting the given values:
n = 0.5 mol,
T = 298 K,
V = 2 L.
P = (0.5 × 8.3145 × 298) / 2 = 623.29 Pascals (Pa)
P = 623.29 Pascals (Pa)
Example 5: Calculate the gas pressure when 4 moles of gas molecules exert a force of 800 Newtons on an area of 100 square meters.
Using the basic pressure formula:
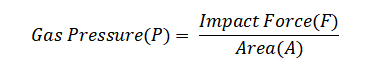
Substituting the provided values:
F = 800 N,
A = 100 m².
P = 800 / 100 = 8 Pascals (Pa)
P = 8 Pascals (Pa)
Example 6: Calculate the gas pressure inside a sealed container with 3 moles of gas at a temperature of 350 Kelvin and a volume of 8 liters.
Using the ideal gas pressure formula:
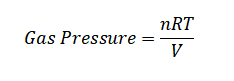
Substituting the given values:
n = 3 mol,
T = 350 K,
V = 8 L.
P = (3 × 8.3145 × 350) / 8 = 1089.1 Pascals (Pa)
P = 1089.1 Pascals (Pa)
These examples serve as practical demonstrations that showcase the application of the respective gas pressure formulas. By considering the provided variables and conditions, individuals can gain a clear understanding of how to calculate gas pressure in various scenarios.
Gas Pressure Formula FAQs
What is gas pressure?
How is gas pressure measured?
What is the ideal gas pressure formula?
What is the SI unit for pressure?
How does temperature affect gas pressure?










