
Gluconic Acid Formula It is an organic compound. It is also one of 16 stereoisomers of 2, 3, 4, 5, 6-pental-hydroxyhexanoic acid. Furthermore, gluconic acid forms the gluconate ion in aqueous solution at a neutral pH. In addition, we know the salts of gluconic acid as 'gluconates'. Gluconates are also used to inject drugs into the body.
Gluconic Acid Formula And Structure
This chemical compound has the molecular formula C6H12O7 and the condensed structural formula HOCH2(CHOH)4COOH. Its chemical structure consists of six carbon chains with five hydroxyl groups (OH), which are arranged in the same manner as in the open chained form of glucose, ending in a carboxylic acid group. Gluconic acid is also in equilibrium with the cyclic ester of gluconic delta-lactone in aqueous solution. The molar mass of the ester is 196.16 g/mol.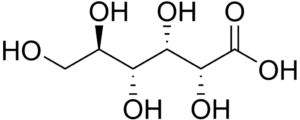
Occurrence of Gluconic Acid
As the result of glucose oxidation, gluconate salts, gluconate esters, and gluconic acid are widely found in nature. In 1929, Horace Terhune Herrick developed a method that could produce its salts by fermentation. However, gluconic acid naturally occurs in fruits, honey, and wine. In addition to acting as an acidity regulator, it is also a food additive (E574).Also Check - Hexane Formula
Preparation of Gluconic Acid
A carboxylic acid that forms naturally in honey, wine, fruits, and many other foods is gluconic acid. It is a nontoxic compound that can be found in nature. A mixture of bromide and sulfuric acid can also be used to synthesize glucose, which is a carboxylic acid. In addition, it can also be prepared by gamma irradiation of D-glucose with a bromine water solution. Oxidation of glucose in bromine water yields glucose acid. Gluconic acid production relies on the fermentative oxidation of glucose's aldehyde group obtained from corn. This process is carried out by various fungi including Aspergillus niger, Aspergillus fumaricus, and Penicillium chryrosogenum. In addition to this method, gluconic acid and sorbitol can also be formed through the Cannizaro reaction using glucose under alkaline conditions. Another option for its preparation is through the electrolytic oxidation of glucose in an alkaline environment. Alternatively, it can be chemically oxidized using a hypochlorite or hypobromite solution or directly oxidized with a palladium catalyst present.Also Check - Azelaic Acid Formula
Physical Properties of Gluconic Acid
| Chemical Formula | C6H12O7 |
| IUPAC Name | D-Gluconic acid |
| Systematic IUPAC name | (2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanoic acid |
| Molar mass | 196.155 g/mol |
| Appearance | Colourless crystals |
| Taste | Mild acid taste |
| Density | 1.23 mg/mL |
| Melting point | 131 °C |
| Solubility | Soluble in water, slightly soluble in alcohol, and insoluble in ether |
| Acidity (pKa) | 3.86 |
| Refractive index | 1.4161 |
| Hydrogen bond donor count | 6 |
| Hydrogen bond acceptor count | 7 |
| Rotatable Bond Count | 5 |
Chemical Properties of Gluconic Acid
As a result of neutralizing gluconic acid with lime or calcium carbonate, calcium gluconate is formed. An aqueous solution of ferrous carbonate and gluconic acid can be used to produce ferrous gluconate or iron (II) gluconate. In water, gluconic acid partially forms an equilibrium mixture with gamma and delta gluconolactone.Also Check - Sugar Formula
Uses of Gluconic Acid
- Gluconic acid has a wide range of applications, such as in industrial cleaning, textile bleach stabilization, aluminum processing, and as a chelating agent for cement set retarding.
- It is also utilized in metal surface treatment, cleaning and personal care products, pharmaceuticals, and as a food additive.
- In addition, calcium gluconate is administered to patients with hypocalcemia while its gel form is used in treating burns caused by hydrofluoric acid. Another gluconic acid derivative, quinine gluconate, is commonly used to treat malaria.
- Ferrous gluconate (also known as iron (II) gluconate) injections have been considered as a potential solution for anemia resulting from iron deficiency.
- To top it off, the aqueous solution of gluconic acid serves as an effective medium for organic synthesis.
Gluconic Acid Formula FAQs
What is the chemical formula of gluconic acid?
The chemical formula of gluconic acid is C6H12O7.
Is gluconic acid a natural compound?
Yes, gluconic acid is a naturally occurring organic acid found in various fruits, honey, and wine.
What is the primary use of gluconic acid?
Gluconic acid is commonly used as a food additive and in cleaning products due to its chelating and mild acidic properties.
Is gluconic acid safe for consumption?
The FDA generally recognizes gluconic acid as safe (GRAS) when used in food and beverage products within specified limits.
How does gluconic acid contribute to its chelating properties?
Gluconic acid forms stable complexes with metal ions, making it effective in removing mineral deposits and stains, which is why it's used in cleaning agents.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2025 Physicswallah Limited All rights reserved.









