
Hexane Formula , Hexane a type of alkane, has a chemical formula of C 6 H 14 and can exist in five different structural isomers or as a combination of them. It is an important component found in gasoline and is primarily extracted through the refining of crude oil. The composition of this fraction varies based on the origin of the oil and the limitations of the refining process. This brief article will delve into the formula, properties, and chemical structure of hexane, as well as its applications.
Properties of Hexane Formula
|
Hexane Properties |
|
| Name | Hexane |
| Appearance | Clear colourless liquid |
| Molecular Formula | C 6 H 14 |
| Melting Point | –96°C to –94°C |
| Boiling Point | 68.5°C to 69.1°C |
| Density | 0.6066 g mL–1 |
| Molar Mass | 86.18 g/mol |
Chemical Structure of Hexane Formula
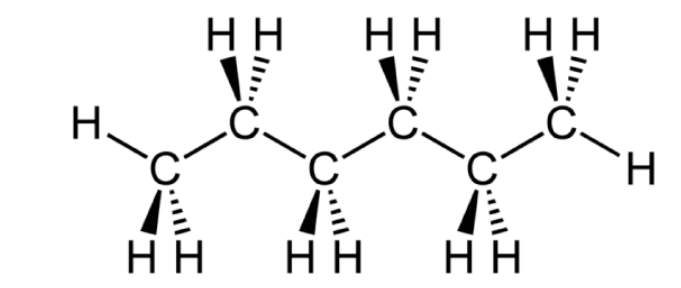
Production of Hexane
In crude oil refining, hexanes are obtained primarily. Their composition is largely determined by the oil source (reformed or crude) and the refining process constraints. In general, the industrial product (around 50% by straight-chain isomer weight) is the fraction boiling at 65-70°C (149-158°F).
Uses of Hexane
In the industrial setting, hexanes have multiple applications. They are utilized in the production of adhesives for shoes, roofing materials, and leather goods. Additionally, they have functional uses, such as extracting cooking oils like soy or canola from seeds, degreasing various objects, and contributing to textile manufacturing. The United States commonly employs hexanes in the extraction of soybean oil and may also be present in other soy-based products due to a lack of FDA regulation. However, this has sparked some controversy.
Also Check – Chemical Bonding Formula
Hexane Safety
Hexane is known for its low acute toxicity. Exposure to 5000 ppm of n-hexane or hexane n for 10 minutes can cause vertigo, while exposure to lower levels of 2500-1000 ppm for 12 hours can result in fatigue, decreased appetite, drowsiness, and numbness in the hands and feet. Higher levels of 2500-5000 ppm may lead to cold pulsations in the extremities, muscle weakness, headache, loss of appetite, and blurred vision.
Occupational exposure to elevated levels of n-hexane or hexane n has been linked to neurotoxicity in printing press workers and peripheral neuropathy in auto mechanics in the United States and factory workers in Europe, Asia, and North America.
There are certain approved uses for n-hexane, such as being a denaturant for alcohol and a cleaning agent in industries like textiles, furniture making, and leather production. However, it's important to note that like gasoline, hexane is highly volatile and poses a risk for explosions.
Also Check – Aluminium Acetate Formula
Intermolecular Forces of Hexane
Hexane exhibits non-polar characteristics for two primary reasons. Firstly, its sole C-H bond has similar electronegativities between hydrogen and carbon, resulting in a non-polar bond. Secondly, the molecule's symmetry prevents any inherent polarity from manifesting. As a result, the only intermolecular forces present in hexane are van der Waals forces/London Dispersion forces or induced dipole-dipole forces. These arise from electrons within hexane repelling those of neighboring hexane molecules, leading to slight positive charges that interact with electron-rich areas within the original molecule.
Also Check – Bleaching Powder Formula
Organic Compounds
Organic compounds are a diverse group of chemical compounds consisting of carbon atoms bonded to other elements such as hydrogen, oxygen, or nitrogen. While carbides, carbonates, and cyanides do not fall under this category, they still contain carbon. These molecules are present in various forms - from living organisms to soils and even everyday items - and play crucial roles in the human body. To understand the importance of carbohydrates, lipids, proteins, and nucleotides in human functioning, it is essential to first understand the chemistry of carbon.
Hexane Formula FAQs
Q1. What is Hexane used for?
Q2. Is Hexane toxic?
Q3. Is Hexane flammable?
Q4. What are the different isomers of Hexane?
Q5. What are the hazards associated with Hexane exposure?










