
Helium gas Formula: In 1895, Sir William Ramsey found helium in London, while Cleve and Nils Langlet independently discovered it in Sweden. Helium's name comes from the Greek word "helios," which means "light," reflecting its discovery near the sun. In 1868, French astronomer Jules Janssen observed the first signs of helium near the sun, and English scientist Edward Frankland coined the term "helium."
Helium Gas Formula
Helium is a safe and non-flammable element, denoted by He with a chemical formula of He gas. It has an atomic number of 2 and is the first noble gas in the periodic table, making it chemically inert. This monatomic gas is colorless, odorless, and tasteless. It was the first gas discovered in the sun and finds widespread applications in various industries. Helium is the second-lightest and one of the most abundant elements in the universe, following only hydrogen.
Helium is called an inert gas because its outer electron orbit holds only two electrons. You can also find helium in lasers, compressed air tanks, and nuclear reactor coolants. It stands out as the element with the lowest boiling and melting points among all the elements. When hydrogen fuses in stars, it produces a lot of helium.
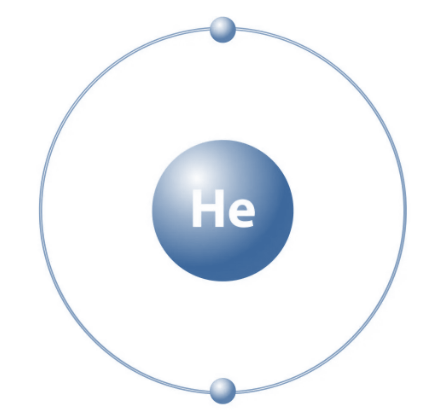
Helium is an element, which means it's made up of just one type of atom, the helium atom. Each helium atom has two protons. If you were to change the number of protons, it would become a completely different element. Helium's atomic number is two because every helium atom contains two protons.
Helium Gas Formula Structure
Helium is usually found as a single atom, not in compounds. Its electron configuration is 1s 2 . The structure of helium gas is like closely packed crystals. Helium belongs to Period 1, Group 18 on the periodic table. When exposed to an electric field, it can turn a red-orange color.
According to the Molecular Orbital Theory, the formula for helium is not He 2 , but in the liquid phase, He 2 can be correct because of the dominant Van der Waals force between helium atoms. Under normal pressure, liquid helium remains in a liquid state regardless of the temperature.
Isotopes of Helium Formula
A helium atom consists of two protons, and the different isotopes of helium vary in the number of neutrons. There are seven known isotopes of helium, ranging from He-3 to He-9. Many of these isotopes have various decay pathways, determined by the nucleus's energy and angular momentum quantum number.
The stable isotopes of helium are 3He and 4He. Helium-3 makes up about 0.0002 percent of helium, while helium-4 accounts for 99.9998 percent. In Earth's atmosphere, the ratio of 4He to 3He atoms is approximately 1,000,000 to 1.
Helium Gas Formula Physical Properties
Helium is a gas that you can't see or smell, and it doesn't catch fire. It's lighter than the air around us. When it's transported, it's so cold that it turns into a liquid, and this extreme cold can freeze other gases. If you touch liquid helium, you can get severe frostbite. Scientists use liquid helium for cryogenic research and as a coolant in nuclear reactors.
- Relative density: Helium is incredibly light; its relative vapor density is just 0.14 (compared to air = 1).
- Viscosity: At 20°C, the viscosity of helium is 1.953.
- Octanol/water partition coefficient: It's 0.28 LogP.
- Boiling point: Helium boils at a super chilly -268.928 degrees Celsius.
- Melting point: It turns into a solid at -272.2°C.
- Solubility: Helium dissolves very little in water, just 0.97 mL per 100 mL at 0°C and 1.08 mL per 100 mL at 50°C. It doesn't dissolve in ethanol either.
Helium Gas Formula Chemical Properties
- Electronic configuration: Helium's electronic configuration is 1s 2 .
- Ionization energy: The first ionization energy of helium is 2372.3 kJ/mol, and the second ionization energy is 5250.5 kJ/mol.
- Vander Waals Radius: The Vander Waals radius of helium is 140 picometers.
- Enthalpy of Fusion: The enthalpy of fusion of helium is 0.0138 kJ/mol.
Uses of Helium
Helium finds various important applications:
Altitude Research and Balloons: It's commonly used in altitude research and meteorological balloons.
Welding: Helium acts as an inert gas shield for welding to protect metals from oxidation.
Cooling: It's the only coolant capable of reaching extremely low temperatures, going below 15K (-434oF).
ability to pass through solids faster than air.
Gas Chromatography: It's used as a carrier gas in gas chromatography, an analytical technique.
Cryogenics: Liquid helium is vital in cryogenic applications, such as superconducting magnets and magnetic resonance imaging (MRI), thanks to its very low melting point.
Semiconductor Manufacturing: Helium plays a role in creating germanium and silicon crystals used in electronics.
Leak Detection: Helium is used for detecting pipeline leaks due to its
| Related Links | |
| Lithium Bromide Formula | Magnesium Sulfate Formula |
| Lithium Hydroxide Formula | Magnesium Oxide Formula |
Helium Gas Formula FAQs
What is the chemical formula of helium gas?
How was helium discovered, and what is the origin of its name?
Is helium a flammable gas?
What is helium's atomic number, and where does it appear on the periodic table?
What is the electron configuration of helium?










