
ICSE Class 10 Chemistry Answer Key 2024: The ICSE Class 10 Chemistry Answer Key 2024 is a valuable resource for students who have taken the exam. It provides them with the correct answers to the questions asked in the ICSE Class 10 Chemistry paper for the year 2024. With the answer key, students can check their responses and evaluate their performance in the exam. This allows them to identify any mistakes they may have made and understand the areas where they need to improve.
The paper is divided into two sections, Section I (40 marks) and Section II (40 marks). Section I comprises compulsory short answer questions covering the entire syllabus. Section II includes six questions, out of which students must answer any four.ICSE Hindi Question Paper 2024 Analysis
The Council for Indian School Certificate Examinations (CISCE) conducted the ICSE Chemistry Paper 2024 on March 11, 2024. The examination commenced at 11:00 a.m. and concluded at 1:00 p.m. Subsequent to the exam, students can access the ICSE Chemistry answer key 2024 and the ICSE Chemistry Question Paper PDF on this page.ICSE Class 10 Chemistry Question Paper 2024 PDF
ICSE Class 10 Chemistry Answer Key Overview
Here is the overview of ICSE Class 10 Chemistry Answer Key:| Particulars | Details |
|---|---|
| Conducting Body | Council for Indian School Certificate Examinations (CISCE) |
| Examination Name | ICSE Class 10 Board Examination |
| Subject | Chemistry |
| Session | 2023-24 |
| ICSE Chemistry answer key 2024 | March 11, 2024 (Will be Available soon) |
| ICSE Class 10th Chemistry Exam date | 11th March, 2024 |
| Total Marks (Theory) | 80 |
| Internal Assessment | 20 |
| Examination Mode | Offline |
| Official Website | www.cisce.org |
ICSE Chemistry Questions Paper 2024 with Solution
The ICSE Chemistry Question Paper 2024, along with its solutions, is a helpful resource for students studying for the exam. It allows students to check their answers and see where they may need improvement. By looking at the solutions, students can learn from their mistakes and better understand the concepts. This helps them feel more confident and prepared for the ICSE Chemistry exam. Having access to the ICSE Chemistry Question Paper 2024 with Solution is important for students to do well in their studies.Chemistry Answer Key 2024- SECTION A
Question 1
Choose the correct answers to the questions from the given options. (Do not copy the questions, write the correct answers only.) (i) Unsaturated hydrocarbons undergo a. Addition reaction (b) Substitution reaction (c) Oxidation reaction (d) Redox reactionAnswer – a. Addition reaction
In the 2nd period Neon has maximum lonization Potential because (a) It has unstable electronic configuration (b) It easily accepts electrons (c) It easily loses electrons. (d)The outer most shell is completely filledAnswer - d. The outer most shell is completely filled
(iii) Copper, Zinc and Tin are the metals alloyed to form: (a) Duralumin (b) Brass (c)Bronze (d) SolderAnswer - c. Bronze
(iv) The metal hydroxide which reacts with both acids and alkalis to form salt and water is: (a) Calcium hydroxide (b) Magnesium hydroxide (c) Aluminium hydroxide (d) Ferric hydroxideAnswer - c. Aluminium hydroxide (ANSWER)
(1) Reaction of an alcohol with a carboxylic acid in the presence of concentrated H₂SO is termed as: (a) Halogenation (b) Esterification (c) Hydrogenation (d) DehydrohalogenationAnswer - b. Esterification
(vi) Conversion of Ethanol to Ethene by the action of concentrated sulphuric acid involves: (a) Dehydration (b) Dehydrogenation (c) Dehydrohalogenation (d) HydrolysisAnswer - a. Dehydration
(vii) The oxidizing agent in the equation S+2H2SO4 3SO2 + 2H2O is (a) Sulphur (b) Sulphuric acid (c) Sulphur dioxide (d) WaterAnswer - b. Sulphuric acid
(vi) Electron Affinity is maximum in (a) Mg (b) Ar (c) la (d)BrAnswer - d. Br
(x) The compound that is not a constituent of the electrolytic mixture used in the Hall-Heroult’s process is (a) AlO (b) NaAlO (c) Na AlF (4) CaFAnswer - b. NaAlO
(x) On passing ammonia gas over heated copper oxide for some time, a reddish-brown residue is left behind. What property of ammonia is demonstrated here? (a) Basic property (b) Oxidising property (c) Redueng property (d) Aerdie propertyAnswer - c. Redueng property
(XI) Rotten et smell is due to the liberation of (a) HCI gas (b) HS (c) Cligas (d) SO gasAnswer - b. HS
(xii) Ammonia gas is collected by downward displacement of air since ammonia is: (a) very slightly soluble in water. (b) heavier than air. (c) lighter than air (d) insoluble in water.Answer - (c) lighter than air.
xiii)

Question 2
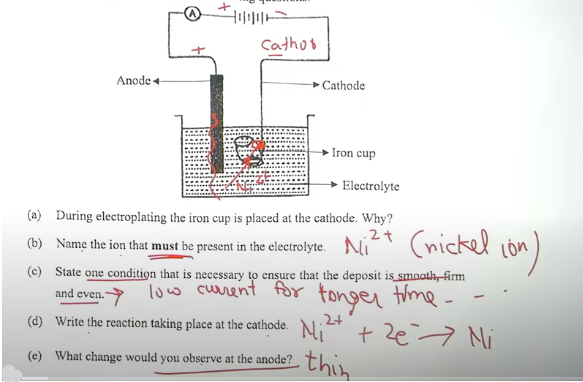
(i) The following sketch represents the electroplating of an Iron cup with Nickel metal. Study the diagram and answer the following questions: [5] Anode Cathode Iron cup Electrolyte (a) During electroplating the iron cup is placed at the cathode. Why? (b) Name the ion that must be present in the electrolyte. (c) State one condition that is necessary to ensure that the deposit is smooth, firm and even. (d) Write the reaction taking place at the cathode (e) What change would you observe at the anode?Answers

(iii) Complete the following sentences by choosing the correct answer from the brackets:
Bold options are answers
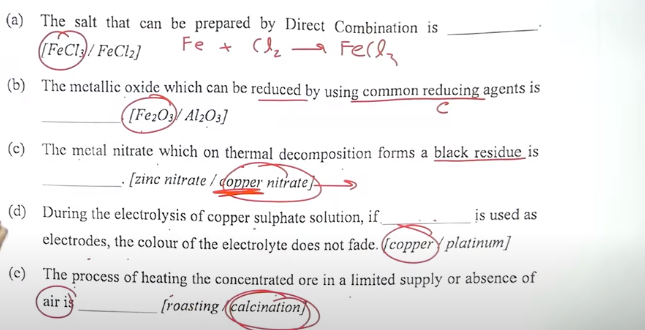
(a) The salt that can be prepared by Direct Combination is —–[FeCl3/FeCl2] (b) The metallic oxide which can be reduced by using common reducing agents is____ [Fe2O3/Al2O3] (c) The metal nitrate which on thermal decomposition forms a black residue is_____ [zinc nitrate/copper nitrate] (d) During the electrolysis of copper sulphate solution, if is used as electrodes, the colour of the electrolyte does not fade___. [copper/platinum] (c) The process of heating the concentrated ore in a limited supply or absence of air is____ [roasting/calcination]Answers
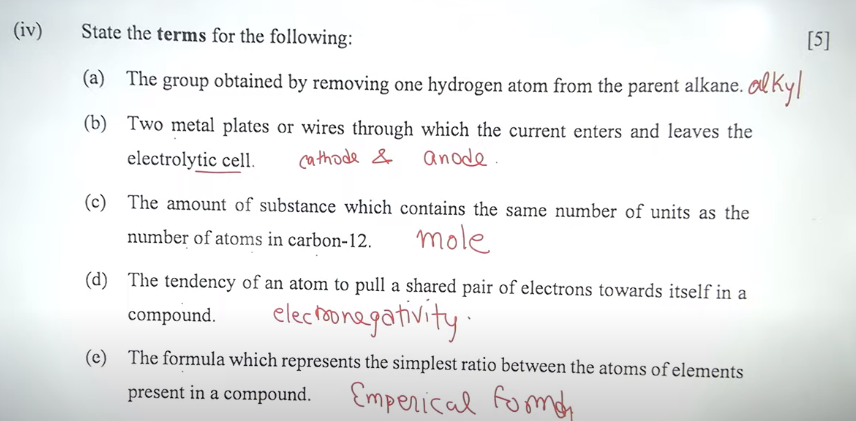 iv) b) Answer – cathode & Anode / Electrodes
iv) b) Answer – cathode & Anode / Electrodes
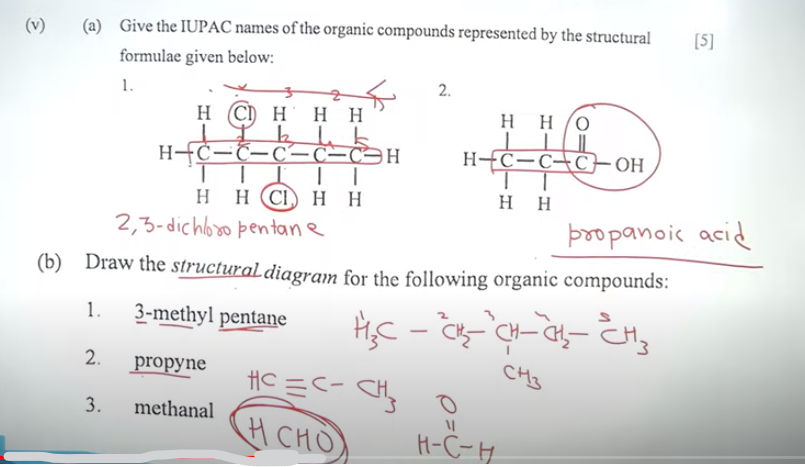
Chemistry Answer Key 2024: SECTION B
Question 3
(i) Rewrite the following statements by adding the correct word as shown in the example: Example: Given Statement: Ammonia changes moist red litmus to blue. Correct Statement: Aqueous ammonia changes moist red litmus to blue. (a) Sulphuric acid acts as a dehydrating agent. (b) Ammonia reacts with chlorine to give ammonium chloride and nitrogen.Answers –
 (II)
(II)

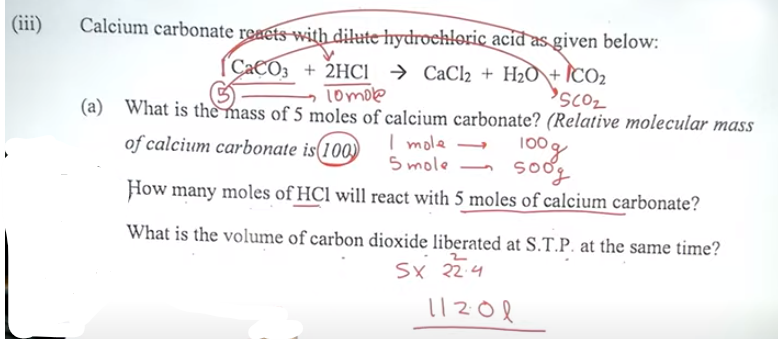
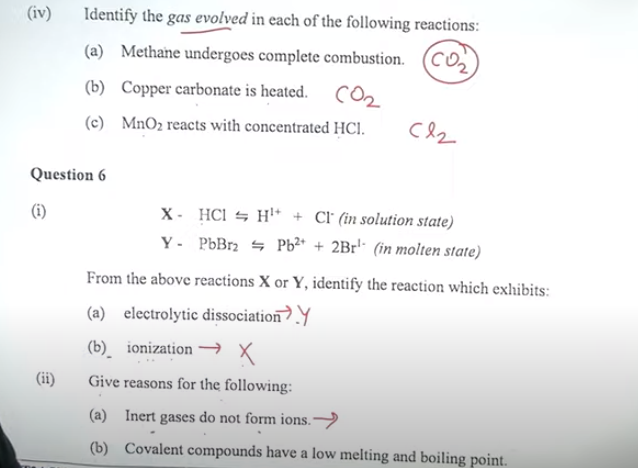
Question 5

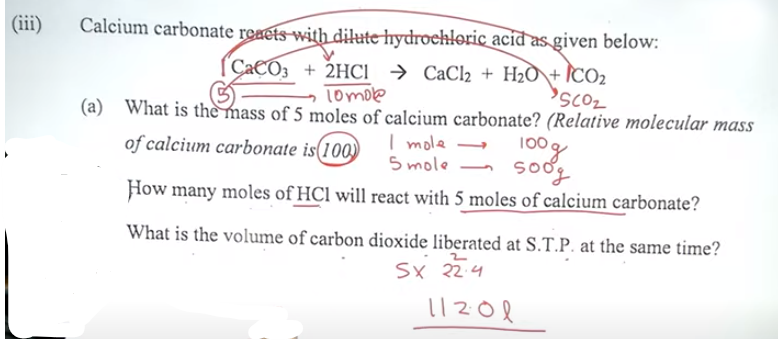
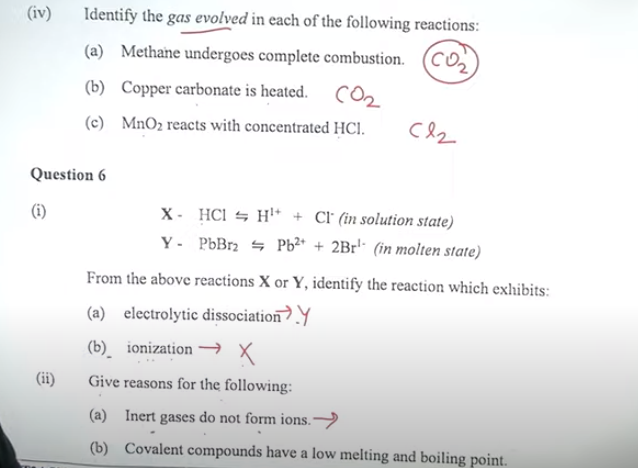
 (ii) Fill in the blanks by choosing the correct answer from the bracket:
(a) Carbon tetrachloride is a ___Non polar____.[polar /
non-pola
r/ covalent molecule].
(b) During electrolysis of acidulated water, the gas liberated at the anode(+)
is__oxygen_____ [oxygen
/ hydrogen].
(ii) Fill in the blanks by choosing the correct answer from the bracket:
(a) Carbon tetrachloride is a ___Non polar____.[polar /
non-pola
r/ covalent molecule].
(b) During electrolysis of acidulated water, the gas liberated at the anode(+)
is__oxygen_____ [oxygen
/ hydrogen].
ICSE Chemistry Answer Key 2024
Here are the first few questions from the ICSE Class 10 Chemistry Question Paper 2024 along with the answer codes. All answers have been accurately verified. If students believe any answer needs correction, they are encouraged to provide feedback below:| Question | Answer |
|---|---|
| i | A. Addition reaction |
| ii | D. The outermost shell is completely filled |
| iii | C. Bronze |
| iv | C. Aluminum hydroxide |
| v | B. Esterification |
| vi | A. Dehydration |
| vii | B. Sulphuric acid |
| viii | D. Br |
| ix | B. NaALO2 |
| x | C. Reducing property |
| xi | B. H₂ S gas |
| xii | C. lighter than air |
| xiii | B. 1 and 3 |
| xiv | C. Two lone pairs of electrons |
| xv | B. Ore |
Match the Column:
| Question Number | Answer |
|---|---|
| a | Water - Covalent compound |
| b | Alkali metal - Lithium |
| c | Halogen - Iodine |
| d | Calcium oxide - Ionic compound |
| e | Weak acid - Acetic acid |
ICSE Class 10 Chemistry Question Paper 2024 PDF
The PDF link for the ICSE Class 10 Chemistry Question Paper 2024 is provided below. This question paper is an essential resource for students preparing for their ICSE Class 10 Chemistry exam. By accessing this PDF, students can familiarize themselves with the format of the exam, practice solving questions, and assess their preparation level.ICSE Class 10 Chemistry Answer Key 2024 FAQs
When will the ICSE Class 10 Chemistry exam held?
What is the full marks of ICSE Chemistry 2024 paper?
How many questions need to attempt in Class 10 Chemistry exam 2024?
Are the answers in the ICSE Class 10 Chemistry Answer Key 2024 accurate?










