Chapter 4 of Class 7 Science, Acids, Bases, and Salts, is crucial for understanding fundamental chemical concepts that are applicable in everyday life. It explains the properties of acids and bases, such as their taste, reactivity, and role in neutralization reactions.
The chapter emphasizes the importance of acids and bases in various natural processes, like digestion and plant growth, and their widespread use in industries, cleaning, and food preservation. By learning about salts, students recognize their significance in areas such as medicine (antacids), agriculture (fertilizers), and cooking (sodium chloride). The chapter also highlights the role of indicators in testing acidity and alkalinity, fostering a deeper understanding of chemical reactions.

Important Questions for Class 7 Science Chapter 4: Chapter 4 of Class 7 Science, Acids, Bases, and Salts, introduces students to the properties and characteristics of acids, bases, and salts. It explores common examples of acids (like vinegar and citrus fruits) and bases (such as soap and baking soda), highlighting their taste, feel, and reactions with indicators like litmus paper.
The chapter also covers the process of neutralization, where an acid reacts with a base to form water and salt. Students learn about the importance of acids, bases, and salts in daily life and their uses in various chemical processes.Important Questions for Class 7 Science Chapter 4 Overview
Important Questions for Class 7 Science Chapter 4 PDF
Below, we have provided a PDF containing important questions for Class 7 Science Chapter 4, Acids, Bases, and Salts. These questions cover key concepts like the properties of acids and bases, their reactions with indicators, and the process of neutralization. The PDF will help you revise and test your understanding of the chapter's core topics, ensuring a strong grasp of essential scientific principles.Important Questions for Class 7 Science Chapter 4 PDF
Important Questions for Class 7 Science Chapter 4 Acids, Bases and Salts
Below we have provided Important Questions for Class 7 Science Chapter 4 Acids, Bases and Salts -Very Short Answer Questions (1 mark)
Fill in the blanks:
1. ______are sour in taste.
Ans: Acids
2. Bases are ______in taste.
Ans: Bitter
3. _______ are soapy to touch.
Ans: Bases
4. __________ are used to determine if a solution is acid or base.
Ans: Indicators
5. Ant bite contains ______________.
Ans: Formic acid.
6. Vinegar contains _________________.
Ans: Acetic acid.
7. Lactic acid is present in _____.
Ans: Curd.
8. Solution which does not change colour in presence of any indicator is considered to be _____________.
Ans: Neutral.
9. Oxalic acid is present in __________.
Ans: Spinach.
10. Phenolphthalein turns pink in ______________.
Ans: Bases.
3 Mark Questions
Short Answer Questions (3 marks)
1. What is litmus? Explain its action.
Ans: One kind of indicator that comes from lichens is litmus. In distilled water, it appears purple. Consequently, under neutral conditions, it is purple. When combined with acids, it turns crimson. When combined with bases, it turns blue. It is available as solutions and strips of red and blue litmus paper.
2. Explain the action of turmeric as an indicator.
Ans: One sign that can be seen in nature is turmeric. In a neutral environment, it is yellow. When exposed to acid, it becomes a deeper shade of yellow. When subjected to simple conditions, it turns scarlet. Turmeric stains on fabrics turn scarlet when they come into touch with soap since it is a basic substance.
3. Explain the use of China rose as an indicator.
Ans: An excellent illustration of a natural indication is the growth of China. In neutral circumstances, it has an orange colour. When exposed to acid, it turns from dark pink to magenta. In simple conditions, it turns green.
4. Explain the use of phenolphthalein as an indicator.
Ans: The marker phenolphthalein is artificial. In neutral conditions it is colourless. Basic conditions cause it to turn pink, while acidic conditions cause it to turn colourless.
5. What is neutralization?
Ans: Neutralisation is the process by which an acid and a base react to form salt and water. In order to reverse the effects of an acid, a base must react with it, and vice versa. The neutralisation process results in the formation of salt and water.
Acid + Base → Salt + water.
3 for 5 Mark Questions
Long Answer Questions (5 marks)
1. Explain the uses of neutralization in daily life.
Ans: In our daily lives, acids and bases are important. Therefore, the other is employed to offset the effect of the first. Examples of common neutralising reactions that we see in daily life include the following:
-
Antacid use: Acidity in the stomach is a result of indigestion. The stomach produces more acid than is necessary in this circumstance. It can be neutralised with basic salts or weak bases. You will feel better after the base neutralises the acid.
-
Ant bite treatment: Ant bites contain formic acid, which causes skin irritation, swelling, and rashes. Basic chemicals such as calamine or baking soda can be used to neutralise the acid in the sting and provide comfort.
-
Soil Treatment: Plants that are growing in an environment with a balanced pH. This condition changes according to the plant. Conversely, plants cannot thrive in extremely acidic or alkaline conditions. Adding acidic organic matter, such compost and manure, can help restore overly alkaline soil. Slaked lime can be used to balance out excessively acidic soil.
-
Treatment of industrial wastes: Acidic chemicals found in industrial wastes are highly concentrated and will destroy aquatic life if they are dumped into water bodies. Therefore, before being released, they need to be neutralised with basic chemicals.
-
Toothpaste: When bacteria in our mouth break down food lodged between our teeth, acidic compounds are formed. Tooth decay is caused by these acids. Toothpaste is so basic in nature in order to neutralise acids in the mouth.
2. What is acid rain?
Ans: Rain or precipitation with an excess of acids is referred to as acid rain. This is brought on by an increase in air pollutants. Rainwater reacts with polluting gases like sulphur, nitrogen oxides, carbon dioxide, and carbon monoxide.
In this downpour, acids predominate. Because acids are corrosive, acid rain is also hazardous. Acid rain corrodes and damages buildings. It damages the skin, which leads to skin issues. Additionally, it causes soil and water bodies to become more acidic, which harms aquatic and plant life.
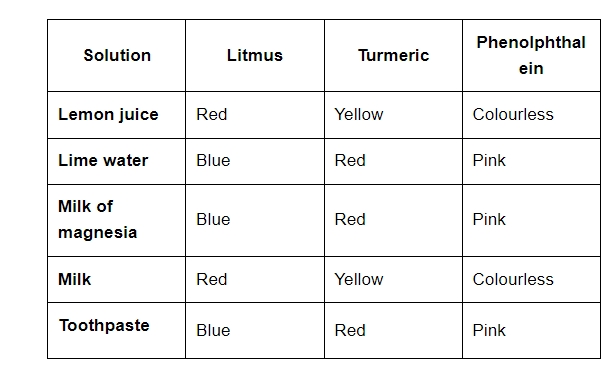
3. There are few solutions given. Compare the reactions to the use of litmus, turmeric and phenolphthalein as an indicator. Lemon juice, Lime water, Milk of magnesia, Milk, Toothpaste
Ans
Benefits of Using Important Questions for Class 7 Science Chapter 4
Using important questions for Class 7 Science Chapter 4, Acids, Bases, and Salts, offers several benefits:Concept Clarity : These questions help reinforce key concepts such as properties, reactions, and uses of acids, bases, and salts.
Focused Revision : By practicing relevant questions, students can focus on critical areas of the chapter, improving retention.
Better Understanding : Answering these questions helps students grasp complex ideas like neutralization and the role of indicators.
Exam Preparation : Regular practice of important questions boosts confidence and prepares students for assessments.
Application in Daily Life : These questions highlight the real-life applications of acids, bases, and salts, making learning more relatable and practical.
Important Questions for Class 7 Science Chapter 4 FAQs
What is the strongest base acid?
The strongest known superacid is fluoroantimonic acid. A superbase is an extremely strong base, that is a compound that has a high affinity for protons. The hydroxide ion is the strongest base possible in aqueous solutions, but bases exist with much greater strengths than can exist in water.
What is common in acid, base, and salt?
Acids and Bases neutralize and form salt and water.
What are the two uses of acid base and salt in everyday life?
Acids, bases and salts have distinctive uses in daily lives, especially in terms of making soaps, fertiliser production, smelting salts and removing warts. Acids, salts and bases have significant real-time uses that enable them to penetrate the basic necessity of human lives successfully.
How to identify acid and base?
There are two types of litmus paper available that can be used to identify acids and bases – red litmus paper and blue litmus paper. Blue litmus paper turns red under acidic conditions and red litmus paper turns blue under basic or alkaline conditions.
🔥 Trending Blogs
Talk to a counsellorHave doubts? Our support team will be happy to assist you!

Check out these Related Articles
Free Learning Resources
PW Books
Notes (Class 10-12)
PW Study Materials
Notes (Class 6-9)
Ncert Solutions
Govt Exams
Class 6th to 12th Online Courses
Govt Job Exams Courses
UPSC Coaching
Defence Exam Coaching
Gate Exam Coaching
Other Exams
Know about Physics Wallah
Physics Wallah is an Indian edtech platform that provides accessible & comprehensive learning experiences to students from Class 6th to postgraduate level. We also provide extensive NCERT solutions, sample paper, NEET, JEE Mains, BITSAT previous year papers & more such resources to students. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app.
We Stand Out because
We provide students with intensive courses with India’s qualified & experienced faculties & mentors. PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We believe in empowering every single student who couldn't dream of a good career in engineering and medical field earlier.
Our Key Focus Areas
Physics Wallah's main focus is to make the learning experience as economical as possible for all students. With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants. From providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation.
What Makes Us Different
Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. Apart from catering students preparing for JEE Mains and NEET, PW also provides study material for each state board like Uttar Pradesh, Bihar, and others
Copyright © 2026 Physicswallah Limited All rights reserved.







