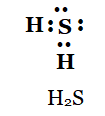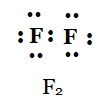NCERT Solutions for Class 10 Science Chapter 4: The NCERT Solutions for Class 10 Science Chapter 4 – Carbon and Its Compounds are a comprehensive and student-friendly resource designed to help learners master one of the most important chapters in the CBSE Class 10 Science syllabus.
These carbon and its compounds Class 10 NCERT Solutions provide detailed explanations for all textbook questions, including in-text and end-of-chapter exercises, ensuring complete coverage of the chapter.
Class 10 Science Chapter 4 Carbon and Its Compounds
NCERT Solutions for Class 10 Science Chapter 4, Carbon and Its Compounds, covers the versatile nature of carbon, its compounds, and their importance in everyday life.
The chapter begins by explaining the unique bonding properties of carbon, including its tetravalency and catenation, allowing it to form a wide variety of organic compounds.
The concept of covalent bonding in carbon is introduced, explaining how carbon forms single, double, and triple bonds with other elements. NCERT Solutions for Class 10 Science Chapter 4 also covers hydrocarbons, which are compounds containing only carbon and hydrogen
Carbon and its Compounds Class 10 Questions with Answers
Carbon and Its Compounds Class 10 Questions with Answers help students practice all important NCERT and exam-oriented questions with clear explanations. These questions and answers are based on carbon and its compounds Class 10 NCERT solutions, making revision easy and effective for board exams.
NCERT Solutions for Class 10 Science Chapter 4 In-text questions set 1
1. What would be the electron dot structure of carbon dioxide which has the formula CO 2 ?
Solution:
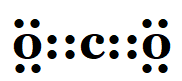
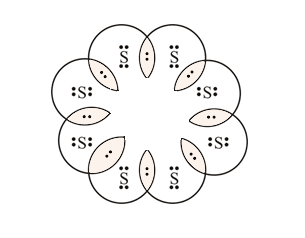
2. What would be the electron dot structure of a molecule of Sulphur which is made up of eight atoms of Sulphur? (Hint – The eight atoms of Sulphur are joined together in the form of a ring).
Solution:
NCERT Solutions for Class 10 Science Chapter 4 In-text questions set 2
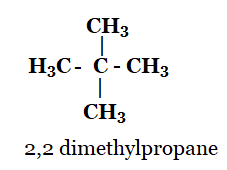
1. How many structural isomers can you draw for pentane?
Solution: The structural isomers of pentane are as follows:
n-pentane 2-methylbutane 2, 2-dimethylpropane 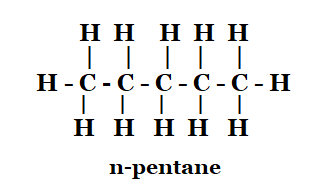
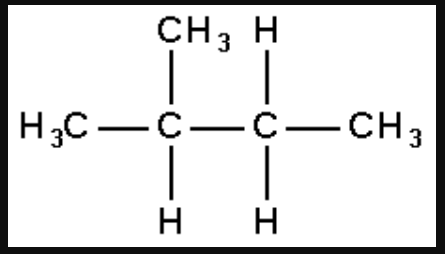
2-methylbutane
2. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Solution:
The following are two characteristics of carbon that contribute to the enormous variety of carbon compounds that surround us:
- Six valence electrons on carbon is actually a large number of valency electrons.
- With carbon atoms and many other atoms, including oxygen, chlorine, nitrogen, sulphur, hydrogen, etc., covalent bonding is simple.
3. What will be the formula and electron dot structure of cyclopentane?
Solution: The formula and electron dot structure of cyclopentane is as given below:
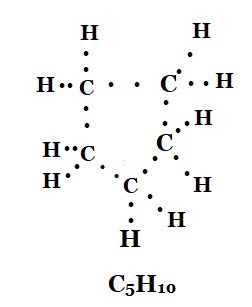
4. Draw the structures for the following compounds.
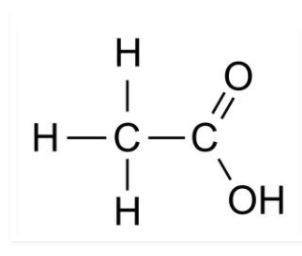
(i) Ethanoic acid
(ii) Bromopentane*
(iii) Butanone
(iv) Hexanal
Solution: i)
ii)
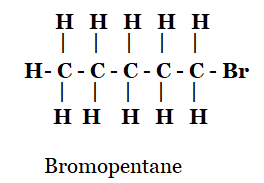
iii)
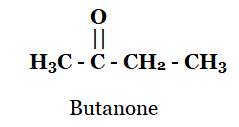
iv)
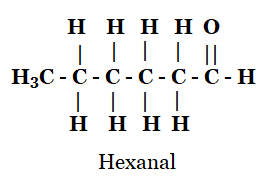
5. How would you name the following compounds?
- CH 3 —CH 2 —Br
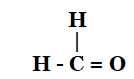
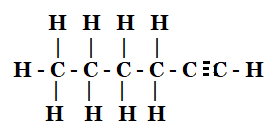
Solution:
-
- Bromoethane
- Methanal or Formaldehyde
- 1 – Hexyne
Class 10 Science Chapter 4 Exercise question Answer In-text questions set 3,
1. How is the conversion of ethanol to ethanoic acid an oxidation reaction?
Solution:
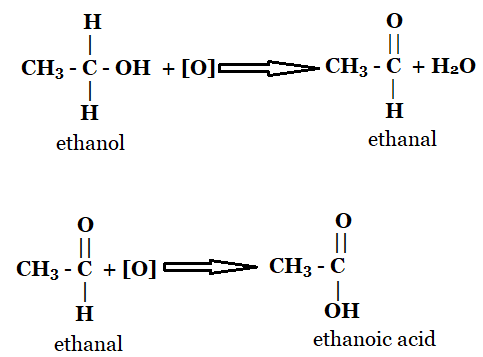 This is an oxidation reaction that turns ethanol into ethanoic acid by taking off the hydrogen atom and adding oxygen. To create ethanal, ethanol must first lose one of its H2 molecules.
A blue flame that is extremely hot when oxygen and ethyne are combined produces a sooty flame, which indicates that certain particles are still unburned and results in less heat.
This is an oxidation reaction that turns ethanol into ethanoic acid by taking off the hydrogen atom and adding oxygen. To create ethanal, ethanol must first lose one of its H2 molecules.
A blue flame that is extremely hot when oxygen and ethyne are combined produces a sooty flame, which indicates that certain particles are still unburned and results in less heat.
NCERT Solutions for Class 10 Science Chapter 4 In-text questions set 4
1. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Solution: In reaction with sodium carbonate, carboxylic acids produce carbon dioxide gas which turns lime water milky, whereas alcohols do not give this reaction. This experiment can be used to distinguish alcohol and carboxylic acid.
The reaction of the carboxylic acid with sodium carbonate: 2CH 3 COOH + Na 2 CO 3 → 2CH 3 COONa + H 2 O + CO 2
2. What are oxidising agents?
Solution:
Compounds that either add oxygen to a molecule or take hydrogen from it are known as oxidising agents. For instance, nitric acid, potassium nitrate, and halogens.
NCERT Solutions for Class 10 Science Chapter 4 In-text questions set 5
1. Would you be able to check if water is hard by using a detergent?
Solution:
It is not possible to determine the hardness of water by employing detergents, which are ammonium salts or sulphonates of long-chain carboxylic acids. They do not react with calcium and magnesium to determine the type of water, in contrast to soaps.
2. People use a variety of methods to wash clothes. Usually, after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Solution:
In order to get clean garments, agitation is required since it helps the soap micelles capture oil, grease, and other contaminants that need to be eliminated. Particles from their surfaces are washed into the water when they are beaten or otherwise disturbed, cleaning the clothing in the process.
Class 10 Science Chapter 4 Exercise Question Answer
1. Ethane, with the molecular formula C 2 H 6, has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Answer: (b) 7 covalent bonds
Solution: Ethane, with the molecular formula C 2 H 6, has 7 covalent bonds.
2. Butanone is a four-carbon compound with the functional group (a) carboxylic acid (b) aldehyde (c) ketone (d) alcohol
Answer: (c) ketone
3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely
(b) the fuel is not burning completely
(c) the fuel is wet
(d) the fuel is burning completely
Answer: (b) the fuel is not burning completely
Solution: While cooking, if the bottom of the vessel is getting blackened on the outside indicates that the fuel is not burning completely.
4. Explain the nature of the covalent bond using the bond formation in CH 3 Cl.
Solution:
Since these reactions cost more energy and cause the system to become unstable, carbon is unable to gain or lose four electrons. As a result, CH3Cl shares its four electrons with carbon atoms or atoms of other elements to complete its octet configuration. Therefore, the bonding present in CH3Cl is covalent in nature. In this case, one electron is needed by each hydrogen atom to complete its duplet, whereas four electrons are needed by carbon to complete its octet. In addition, chlorine needs one electron to finish the octet. Since they are all sharing electrons, carbon makes three bonds with hydrogen and one with chlorine.
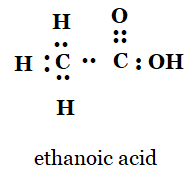
5. Draw the electron dot structures for
(a) ethanoic acid
(b) H 2 S
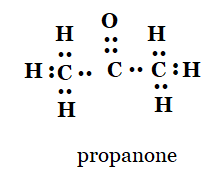
(c) propanone
(d) F 2
Solution:
a)
b)
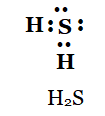
c)
d)
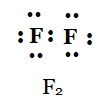
6. What is a homologous series? Explain with an example.
A collection of substances with the same functional group is called a homologous series. The general formula and chemical characteristics of this are likewise comparable. We can state that there would be a rise in molecule mass and size since there is a change in the physical characteristics. For example, methane, ethane, propane, butane, etc., are all part of the alkane homologous series. The general formula of this series is C n H 2n+2 . Methane CH 4 Ethane CH 3 CH 3 Propane CH 3 CH 2 CH 3 Butane CH 3 CH 2 CH 2 CH 3 . It can be noticed that there is a difference of −CH2 unit between each successive compound.
7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Solution:
| Ethanol |
Ethanoic acid |
| It does not react with sodium hydrogen carbonate |
Bubbles and fizzes with sodium hydrogen carbonate |
| A good smell |
Smells like vinegar |
| No action in litmus paper |
Blue litmus paper to red |
| Burning taste |
Sour taste |
8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents, such as ethanol also?
Solution: Micelle formation takes place because of the dirt particles in water and clean water. There are two mediums that are involved: one is pure water, and the other is dirt, also called impurities. The soap also has two mediums:
(i) organic tail (ii) ionic head Thus, the ionic head and oil or grease dissolve and mix with the water, while the organic tail combines and dissolves with the dirt. As a result, the soap molecules in the water pull the dirt away from the object that has to be cleaned. As a result, the micelles' (positively charged heads) reciprocal repulsion causes the soap to form closed structures that clean. Other solvents, including ethanol, are unable to generate these micelles because the sodium salt of fatty acids does not dissolve in it.
9. Why are carbon and its compounds used as fuels for most applications?
Solution: Carbon and its compounds are used as fuels for most applications because they have high calorific values and give out a lot of energy. Most of carbon compounds give a lot of heat and light when burnt in the air.
10. Explain the formation of scum when hard water is treated with soap.
Solution: Scum is produced from the reaction of hard water with soap. Calcium and magnesium present in the hard water form an insoluble precipitate called scum.
11. What change will you observe if you test soap with litmus paper (red and blue)?
Solution: An alkaline solution is produced when soap dissolves in water and forms an alkaline NaOH or KOH solution. The blue litmus stays blue in the soap solution, whereas the red litmus turns blue in the solution.
12. What is hydrogenation? What is its industrial application?
Solution:
A process or chemical reaction involving hydrogen and other substances is known as hydrogenation. Usually, catalysts are present when it is done. For instance, platinum, palladium, or nickel. The primary purpose of hydrogenation is to saturate organic molecules.
13. Which of the following hydrocarbons undergo addition reactions: C 2 H 6 , C 3 H 8 , C 3 H 6 , C 2 H 2 and CH 4?
Solution: Unsaturated hydrocarbons undergo addition reactions. C 3 H 6 and C 2 H 2 are unsaturated hydrocarbons which undergo addition reactions.
14. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Solution:
Alkenes and alkynes are examples of unsaturated chemicals. The bromine water test is used to distinguish between these and other saturated compounds. Bromine is employed in the form of bromine water for this purpose. Bromine water is a solution of bromine in water. Because bromine is present in it, the water is reddish-brown in hue. When an unsaturated compound is mixed with bromine water, the unsaturated molecule absorbs the bromine and the reddish-brown bromine water is released. Hence, an unsaturated hydrocarbon (one that has a double or triple bond) will decolourise bromine water; saturated hydrocarbons, or alkanes, on the other hand, do not decolorise bromine water. To distinguish between saturated and unsaturated chemicals (such as alkenes and alkynes), the bromine water test is used. An unsaturated hydrocarbon releases its reddish-brown bromine solution when bromine water is introduced. Therefore, the chemical will be an unsaturated hydrocarbon if there is discolouration.
15. Explain the mechanism of the cleaning action of soaps.
Solution:
Water is contaminated with a great deal of pollutants and dirt, which doesn't dissolve in the water. Soap molecules consist of a mixture of salts, like potassium and sodium. The molecules are linked carboxylic acids in a lengthy chain.

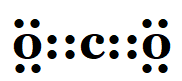

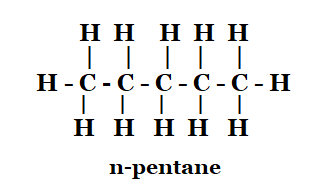




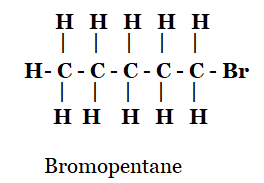
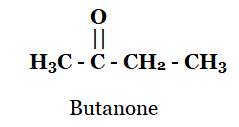
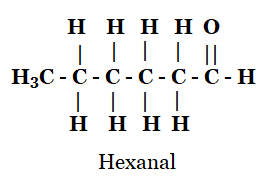
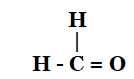
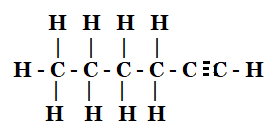
 This is an oxidation reaction that turns ethanol into ethanoic acid by taking off the hydrogen atom and adding oxygen. To create ethanal, ethanol must first lose one of its H2 molecules.
This is an oxidation reaction that turns ethanol into ethanoic acid by taking off the hydrogen atom and adding oxygen. To create ethanal, ethanol must first lose one of its H2 molecules.