
Percent by Weight Formula: Percent by weight, also known as weight percent or weight percentage, is a crucial concept in various fields, including chemistry, physics, engineering, and even everyday life. It is used to represent the concentration of a substance within a mixture or the makeup of a compound by considering its mass concerning the overall mass of the mixture or compound. In this article, we will discuss the formula for computing percent by weight and its real-world uses.
Also Check - Molecular Speed Formula
Percent by Weight Formula
The formula for calculating percent by weight is relatively straightforward and can be expressed as:
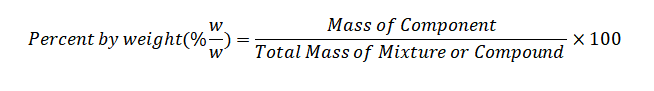
Here's a breakdown of the components of this formula:
Mass of Component: This is the mass or weight of the specific substance you are interested in within the mixture or compound. It is often expressed in grams (g) or kilograms (kg) for scientific applications.
Total Mass of Mixture or Compound: This refers to the combined mass of all substances in the mixture or compound. Again, it is usually measured in grams or kilograms.
Multiplication by 100 : To express the result as a percentage, we multiply the fraction by 100.
Also Check - Mole Fraction FormulaPercent by Weight Practical Applications
Chemistry: Percent by weight is widely used in chemistry to describe the concentration of a solute in a solution. For example, when preparing a saline solution for medical use, the percent by weight of salt (sodium chloride) in the solution must be carefully controlled to ensure it meets the required standards.
Pharmaceuticals: In the pharmaceutical industry, percent by weight is crucial for dosing medications accurately. It helps in determining the concentration of active ingredients in tablets, capsules, and liquid medications.
Food Industry: Food manufacturers use percent by weight to specify the nutritional content of products. For instance, the nutrition label on a cereal box may indicate the percent by weight of vitamins, minerals, or protein in each serving.
Metallurgy: In metallurgy, percent by weight is used to describe the composition of alloys. Engineers need to know the exact composition of metals in alloys to design materials with specific properties, such as strength and corrosion resistance.
Environmental Science: Environmental scientists use percent by weight to analyze pollutants in air, water, and soil samples. By knowing the concentration of pollutants, they can assess environmental impact and make informed decisions regarding remediation efforts.
Construction and Engineering: Civil engineers and construction professionals use percent by weight to determine the mix ratios of concrete, asphalt, and other construction materials. It ensures that the final product meets structural and safety standards.
Manufacturing: In manufacturing processes, especially in quality control, percent by weight is crucial. It helps ensure that products meet the desired specifications and are consistent in composition.
Also Check - Mole Fraction FormulaPercent by Weight Formula Solved Example
Let's work through a solved example to demonstrate how to calculate percent by weight using the formula.
Example: In a solution of saltwater, and you want to find the percent by weight of salt (sodium chloride) in the solution. You weigh 75 grams of the saltwater solution, and after evaporating all the water, you find that the remaining mass of salt is 15 grams. Calculate the percent by weight of salt in the solution.
Solution:
Identify the Given Values:
Mass of the saltwater solution = 75 grams
Mass of the remaining salt after evaporation = 15 grams
Apply the Percent by Weight Formula:
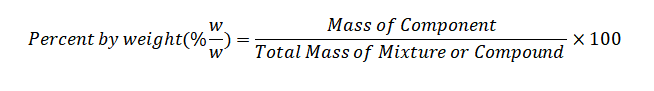
In this case, the "Mass of Component" is the mass of salt (15 grams), and the "Total Mass of Mixture or Compound" is the mass of the saltwater solution (75 grams).
Calculate the Percent by Weight:
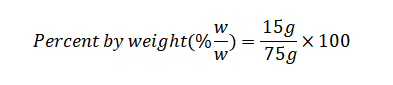
Now, perform the calculation:
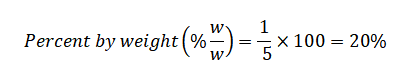
Answer: The salt's percent by weight in the saltwater solution is 20%. This implies that out of every 100 grams of the solution, 20 grams consist of sodium chloride.
This example illustrates how you can use the percent by weight formula to determine the concentration of a specific component within a mixture or solution. In this case, it's the concentration of salt in the saltwater solution, expressed as a percentage.
Percent by weight is a fundamental concept in various scientific and industrial fields. It allows us to express the concentration or composition of substances in mixtures and compounds accurately. Whether you are a chemist, engineer, pharmacist, or anyone dealing with mixtures and compounds, understanding and applying the percent by weight formula is essential for accurate measurements and quality control in your respective fields.
Percent by Weight Formula FAQs
What is percent by weight?
What is the unit of measurement for percent by weight?
Where is percent by weight commonly used?
Can percent by weight exceed 100%?










