
Sodium Hydrogen Phosphate: This article discusses the formula for Sodium hydrogen phosphate, also known as Sodium phosphate dibasic. Its chemical makeup is Na 2 HPO 4 and it can be obtained by combining phosphoric acid (H 3 PO 4 ) with sodium hydroxide (NaOH). In its anhydrous state, it presents as a white, odorless powder that is hygroscopic and has a salty taste. When hydrated, it forms a white crystalline solid with no scent. This inorganic compound is soluble in water but not in alcohol. The process of obtaining it on an industrial level involves two steps.
Sodium Hydrogen Phosphate
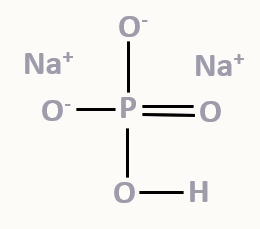
Sodium hydrogen phosphate, also known as disodium hydrogen phosphate, is a salt of phosphoric acid. Its chemical formula, Na 2 HPO 4 , signifies its composition, with two sodium (Na+) ions, one hydrogen (H+) ion, and one phosphate (PO4 3- ) ion. With a chemical formula of Na 2 HPO 4 , sodium hydrogen phosphate is an inorganic and hygroscopic compound. It has a saline taste. It appears in anhydrous form as a white, odorless powder. It appears in hydrated form as a white, odorless crystal. There are other names for Sodium hydrogen phosphate formula, including Disodium phosphate and Sodium phosphate dibasic formula. Sodium hydrogen phosphate is soluble in water, but insoluble in alcohol.
Sodium Hydrogen Phosphate Molecular Weight
The molecular weight of sodium hydrogen phosphate (Na 2 HPO 4 ) is approximately 141.96 grams per mole. This value is calculated by summing the atomic weights of its constituent elements, including sodium, hydrogen, phosphorus, and oxygen.
Sodium Hydrogen Phosphate Dihydrate
Sodium hydrogen phosphate can exist in different forms, including the dihydrate. The dihydrate contains two molecules of water (H 2 O) per molecule of Na 2 HPO 4 . This form often finds use in various applications, including as a laboratory reagent and in the food industry.
Properties of Sodium Hydrogen Phosphate Formula
| Properties of Sodium Hydrogen Phosphate Formula | |
| Chemical formula | Na 2 HPO 4 |
| Molecular weight | 141.96 g/mol (anhydrous) |
| Density | 1.7 g/cm3 |
| Appearance | Crystalline solid |
| Melting point | 250 °C |
Also Check – Internal Energy Formula
Sodium Hydrogen Phosphate Uses
- Food and Beverage Industry: Sodium hydrogen phosphate is used as a food additive and pH regulator in producing various processed foods and beverages.
- Laboratory Reagent: It serves as a valuable reagent in chemical laboratories for various experiments and reactions.
- Buffering Agent: Due to its ability to maintain a stable pH in solutions, it is employed as a buffering agent in chemical and biological research.
- Pharmaceuticals: Sodium hydrogen phosphate is used in the manufacture of pharmaceuticals to control the pH and enhance the stability of certain drug formulations.
- Water Treatment : In water treatment processes, it can be used to adjust the pH of water to specific levels.
Sodium Hydrogen Phosphate pH
Sodium hydrogen phosphate plays a critical role in pH regulation. It acts as a buffer, helping to maintain the pH of a solution close to a particular value. The pH depends on the specific concentration and the ratio of sodium hydrogen phosphate to its conjugate acid, sodium dihydrogen phosphate
Also Check – Ionization Energy Formula .
Sodium Dihydrogen Phosphate Monohydrate
Sodium dihydrogen phosphate monohydrate (Na 2 HPO 4 ·H 2 O) is a related compound to sodium hydrogen phosphate. It contains one molecule of water per molecule of sodium dihydrogen phosphate. This compound is often used as a pH regulator and buffering agent in various applications.
Also Check – Mass Percent Formula
Sodium Hydrogen Phosphate: Acid or Base?
Sodium hydrogen phosphate can act as both an acid and a base, depending on the context. It is amphiprotic, meaning it can either donate or accept protons (H+ ions) in chemical reactions. In acidic environments, it can act as a base by accepting protons, while in basic environments, it can act as an acid by donating protons. This versatility makes it invaluable in pH control and buffering systems.
Sodium Hydrogen Phosphate Formula FAQs
Q1. How do you name Sodium hydrogen phosphate?
Q2. What do sodium and hydrogen phosphate make?
Q3. What is sodium plus hydrogen phosphate?
Q4. Why Sodium hydrogen phosphate?










