
Structural Formula: A structural formula is a symbolic representation of a chemical compound that provides information about the arrangement of atoms and chemical bonds within the molecule. It is a crucial tool for chemists to understand and communicate the structure of compounds. In this comprehensive guide, we will delve into structural formulas, their types, and provide examples to illustrate their importance in the world of chemistry.
Also Check – Aluminium Acetate Formula
Basics of Structural Formulas
At its core, a structural formula is a visual representation of a molecule's structure, showing how atoms are connected and the types of bonds between them. It goes beyond a molecular formula, which only provides the ratio of atoms in a compound. A structural formula gives us precise information about the arrangement of atoms in a molecule.
Also Check – Chemical Bonding Formula
Types of Structural Formulas
There are several types of structural formulas used in chemistry, each providing a different level of detail about a molecule's structure. Here are the most common types:
1.Lewis Structure
A Lewis structure uses dots and lines to represent atoms and bonds, respectively. It shows the valence electrons of each atom, making it useful for understanding the electronic configuration of a molecule. Let's consider water (H2O) as an example:
In this Lewis structure, each line represents a covalent bond, and each dot represents a valence electron. Oxygen (O) has two lone pairs of electrons and forms two covalent bonds with hydrogen (H) atoms.
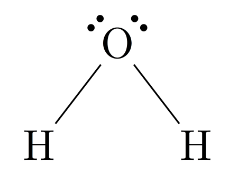
2.Condensed Structural Formula
Condensed structural formulas are a more compact way to represent molecules by omitting certain details. Atoms and their connections are shown, but carbon-hydrogen (C-H) bonds are usually left out for simplicity. An example is ethene (C2H4):
In this formula, carbon atoms are implied at the ends and in the middle of the lines, and hydrogen atoms bonded to carbon are not explicitly shown.
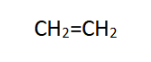
3. Skeletal Formula
Skeletal formulas, also known as line-angle formulas, emphasize the carbon backbone of organic molecules while simplifying the representation of hydrogen atoms and other substituents. For example, consider the skeletal formula for ethanol (C2H5OH):
In this diagram, each line represents a single bond, and carbon atoms are placed at the ends and corners of lines.
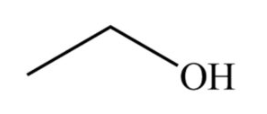
Also Check – Bleaching Powder Formula
Importance of Structural Formulas
Structural formulas are essential in chemistry for several reasons:
1 Determining Chemical Properties
The arrangement of atoms and bonds directly impacts a molecule's chemical properties. By examining the structural formula, chemists can predict how a compound will behave in reactions, its reactivity, and its physical characteristics.
2 Drug Discovery
In pharmaceutical research, structural formulas are used to design and analyze drugs. Understanding a drug's structure allows researchers to modify it for improved efficacy and reduced side effects.
3 Environmental Chemistry
In environmental chemistry, structural formulas help assess the impact of pollutants on ecosystems. By knowing the structure of a pollutant, scientists can predict its behavior in the environment and develop remediation strategies.
4 Material Science
Materials scientists use structural formulas to design and synthesize new materials with specific properties. For example, the structure of polymers determines their strength and flexibility.
Also Check – Surface Chemistry Formula
Examples of Structural Formulas
Let's explore a few examples of structural formulas for common compounds:
1 Methane (CH4)
Methane, the primary component of natural gas, consists of one carbon atom bonded to four hydrogen atoms. Its structural formula is:
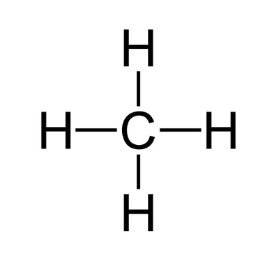
2 Ethanol (C2H5OH)
Ethanol, found in alcoholic beverages, consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. Its condensed structural formula is:
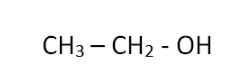
3 Ethene (C2H4)
Ethene, a simple hydrocarbon, contains two carbon atoms double-bonded to each other and two hydrogen atoms bonded to each carbon. Its condensed structural formula is:
H2C=CH2
Structural formulas are indispensable tools in chemistry, enabling scientists to understand and manipulate the structures of molecules. They play a pivotal role in drug discovery, environmental analysis, material science, and countless other areas of research. Whether represented as Lewis structures, condensed structural formulas, or skeletal formulas, these graphical representations provide insight into the world of atoms and molecules, allowing chemists to uncover the secrets of matter and design new compounds for a wide range of applications.
Structural Formula FAQs
What is a structural formula?
Why are structural formulas important in chemistry?
What's the difference between a structural formula and a molecular formula?
What are the different types of structural formulas?
How do you represent covalent bonds in a structural formula?










