
Aluminum acetate formula , with the chemical formula Al (C H 3 C O 2 ) 3, showcases aluminum's remarkable bonding abilities and the acetic acid molecule. This intricate chemistry formula comprises one aluminum atom bonded to three acetic acid molecules. Each acetic acid molecule acts as a ligand, forming coordination bonds with the central aluminum ion through their oxygen atoms. The result is a complex structure where the positively charged aluminum cation resides at the core, surrounded by six negatively charged oxygen atoms from each acetate group. These strong bonds ensure stability and contribute to its applications in pharmaceuticals, textiles, and cosmetics industries. Understanding this chemistry formula allows scientists and researchers to manipulate aluminum acetate's properties effectively for specific purposes like medication delivery systems or fabric dye fixation processes.
Also read : Chemistry Formulas
Aluminum Acetate Formula
[caption id="attachment_14171" align="aligncenter" width="300"]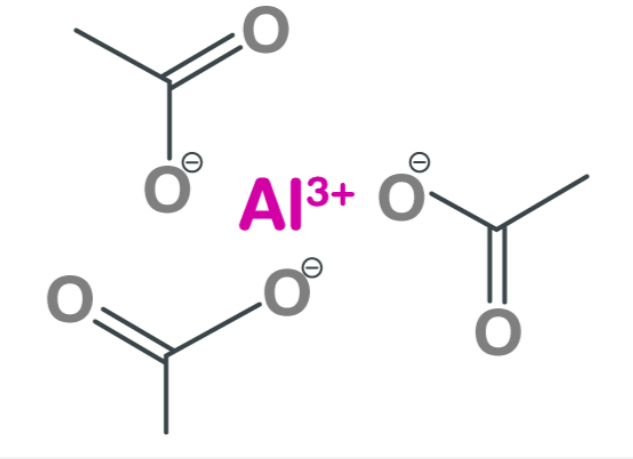 Aluminum Acetate Formula[/caption]
Aluminum Acetate Formula[/caption]
Aluminum acetate exists in three distinct forms as salts. The first one is called "Neutral Aluminum Triacetate," which is commonly known as aluminum acetate. Its chemical formula is Al (C H 3 C O 2 ) 3 .
The second form is "Basic Aluminum Diacetate," which appears as a white crystalline solid and is soluble in water. Its chemical formula is HOAl(CH3CO2)2. This compound is often utilized in over-the-counter topical medications for various skin conditions due to its mild antiseptic and astringent properties.
The third form is "Basic Aluminum Monoacetate," represented by the chemical formula HO2AlCH3CO2. This solid white powder is also called dibasic aluminum monoacetate and is used primarily as an astringent or antiseptic agent.
These variations of aluminum acetate salts serve different purposes in various applications.
Also Check - Hydrocyanic Acid Formula
Aluminum Acetate Structure
Aluminum acetate consists of an aluminum atom in the middle of a chain of acetate ions connected by oxygen atoms. Each acetate ion has its carbon atom linked to two oxygen atoms and a hydrogen atom. It is bonded to two further acetyl groups, each containing a carbon atom with two oxygen atoms and two hydrogen atoms. This structure provides an easily visualized representation of aluminum acetate's composition.
Forms of Aluminum Acetate
Aluminum acetate monohydrate
It is often used as an astringent and antiseptic in topical products to treat minor cuts and abrasions. Aluminum acetate is a white, crystalline powder that dissolves in water.
Aluminum acetate anhydrous
It is a white, crystalline powder soluble in water that is used similarly to aluminum acetate monohydrate but may be more effective in drying out and healing skin irritations.
Aluminum acetate Solution
As a topical skin cleanser or rinse for minor skin irritations and wounds, aluminum acetate solution is a liquid form of aluminum acetate prepared by dissolving aluminum acetate monohydrate or anhydrous in water.
Aluminum acetate Flakes
Soaps and detergents are often made using these thin, flat flakes of aluminum acetate.
Also Check - Hydrocyanic Acid Formula
Aluminum Acetate Precipitation
- Aluminum acetate can be prepared through various methods. One common approach involves mixing aluminum oxide and acetic acid in a 1:3 ratio at room temperature, resulting in aluminum acetate according to the general reaction:
Al2O3 + 3CH3COOH → C6H9(Al)O6
- Alternatively, aluminum acetate can be obtained through decomposition reactions. When aluminum acetate reacts with sodium carbonate, it forms sodium acetate and aluminum carbonate:
Al (C H 3 C O 2 ) 3 + Na2CO3 → Al2(CO3)3 + 2NaC2H3O2
- Similarly, the reaction of aluminum acetate with sodium hydroxide yields sodium acetate and aluminum hydroxide:
Al (C H 3 C O 2 ) 3 + NaOH → C2H3NaO2 + Al(OH)3
- Additionally, aluminum acetate can be synthesized via the electrolytic method, where aluminum metal is dissolved in an aqueous acetic acid solution, producing aluminum acetate:
Al + 2CH3COOH + 2H2O → Al (C H 3 C O 2 ) 3 + 3H2
- The direct synthesis method involves reacting aluminum oxide or hydroxide with acetic acid, resulting in aluminum acetate:
Al2O3 + 3CH3COOH → 2 Al (C H 3 C O 2 ) 3 + 3H2O
2Al(OH)3 + 3CH3COOH → 2 Al (C H 3 C O 2 ) 3 + 6H2O
Also Check - Hexane Formula
Aluminum Acetate Properties
Chemical Formula: Aluminum acetate has the chemical formula Al (C H 3 C O 2 ) 3 , indicating its composition of aluminum (Al) and acetate ions (CH3COO-).
Appearance: It typically appears as a white or colorless crystalline solid.
Solubility: Aluminum acetate is soluble in water and forms solutions that may be used for various applications.
Astringent Properties: Due to its astringent nature, aluminum acetate is commonly used in topical medications to treat skin conditions such as dermatitis, insect bites, and poison ivy rashes.
Antiseptic exhibits mild antiseptic properties, making it valuable in some healthcare products.
Acidic Nature: Aluminum acetate is slightly acidic when dissolved in water.
Decomposition: It can decompose into aluminum carbonate and acetic acid when heated or exposed to certain conditions.
pH Regulation: It can be used in some industries to help control the pH of solutions.
Hygroscopic: Aluminum acetate has hygroscopic properties, meaning it can absorb environmental moisture.
Medicinal Applications: Besides dermatological treatments, aluminum acetate is sometimes used in otic (ear) solutions and certain antiperspirant formulations.
Aluminum Acetate Uses
Dermatological Treatments: Aluminum acetate is widely used in over-the-counter and prescription topical medications to treat skin conditions such as dermatitis, eczema, poison ivy rashes, and insect bites. It helps relieve itching, reduce inflammation, and soothe irritated skin.
Antiperspirants: Some antiperspirant products contain aluminum acetate to help reduce perspiration by constricting sweat glands and providing astringent effects, thus minimizing body odor.
Otic Solutions: Aluminum acetate may be included in ear drops and solutions for removing earwax and treating minor ear infections.
pH Regulation: It is employed in laboratories and industrial settings to control the pH of solutions, making it valuable in processes where pH adjustment is critical.
Hydrocarbon Testing: In the petroleum industry, aluminum acetate is sometimes used to test the hydrocarbon content of drilling mud and oilfield waters.
Wound Care: In some cases, aluminum acetate solutions are used to cleanse and disinfect wounds or ulcers.
Cosmetics: It may be found in cosmetic products, especially those designed for skincare, to provide astringent and skin-conditioning properties.
Leather Tanning: Aluminum acetate can be used in leather tanning processes to help soften and stabilize leather.
Photography: In traditional photographic development processes, aluminum acetate was used as a chemical fixer.
Laboratory and Research: Aluminum acetate is employed as a reagent in various laboratory experiments and chemical research.
Aluminum Acetate FAQs
What is aluminum acetate used for in skincare?
Is aluminum acetate safe for skin applications?
How does aluminum acetate work as an astringent?
Can aluminum acetate be used for earwax removal?
Are there any potential side effects of aluminum acetate in skincare products?










