
TFA Formula: Trifluoroacetic acid is composed of four elemental constituents: Carbon (C), Fluorine (F), Oxygen (O), and Hydrogen (H). Carbon is a nonmetallic element found in group 14 of the periodic table, characterized by an atomic number of six and symbolized as C. Fluorine, the most electronegative element, exists as a highly toxic diatomic gas with a pale yellow hue. It belongs to group 17 of the periodic table, denoted by an atomic number of nine and represented by the symbol F. Oxygen is another nonmetallic element, known for its high reactivity, and is part of the chalcogen group in group 16. It has an atomic number of eight and is symbolized as O. Hydrogen, the lightest among elements, presents as a colorless, odorless, tasteless, and flammable gas. It bears an atomic number of one and is represented by the symbol H.
Also Check – Vapor Pressure Formula
TFA Formula
Trifluoroacetic acid, often abbreviated as TFA, is a potent and colorless fuming liquid with a distinct pungent odor. Chemically, its formula is CF 3 CO 2 H. The synthesis of Trifluoroacetic acid (TFA) involves the treatment of acetyl chloride (CH 3 COCl) through the process of electrochemical fluorination. TFA is widely employed in acid-catalyzed reactions, particularly in cases where ester cleavage is necessary during peptide synthesis. Additionally, it goes by the name Trifluoroethanoic acid and serves as the conjugate acid of a trifluoroacetate. TFA exhibits solubility in water and possesses a density greater than that of water. Its IUPAC nomenclature is 2,2,2-trifluoroacetic acid.
There are two methods for preparing Trifluoroacetic acid:
The conventional approach entails heating hexachloropropene with AgF to produce trichloro trifluoropropene, which is then further oxidized with potassium permanganate to yield Trifluoroacetic acid.
An alternative method involves the electrochemical fluorination of acetyl chloride or acetic acid using the Simons method, followed by the subsequent hydrolysis of trifluoroacetyl fluoride via the reaction:
CH 3 COCl + 4HF → CF 3 COF + 3H 2 + H + + Cl –
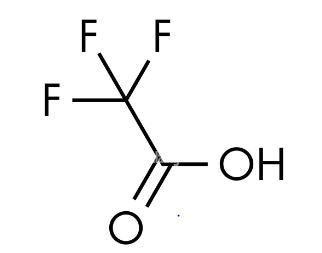
The Structure of Trifluoroacetic Acid
Trifluoroacetic acid exhibits a particular molecular arrangement, characterized by its chemical composition and three-dimensional configuration. The molecular structure of Trifluoroacetic acid plays a pivotal role in understanding its physical and chemical properties, as well as its various applications.
Physical Properties of Trifluoroacetic Acid
Molecular Weight: The molecular weight of Trifluoroacetic acid is approximately 114.023 grams per mole (g/mol). This parameter is crucial for understanding its mass and stoichiometry in chemical reactions.
Density: Trifluoroacetic acid, often abbreviated as TFA, possesses a density of approximately 1.489 grams per milliliter (g/mL). This density value indicates its relative heaviness compared to water.
Melting Point: Trifluoroacetic acid exhibits a melting point of -15.4 degrees Celsius (-15.4°C). This property signifies the temperature at which it transitions from a solid to a liquid state.
Boiling Point: The boiling point of Trifluoroacetic acid is approximately 72.4 degrees Celsius (72.4°C). This temperature marks the point at which the substance changes from a liquid to a vapor when exposed to heat.
Enthalpy of Vaporization: Trifluoroacetic acid has an enthalpy of vaporization of approximately 33 kilojoules per mole (kJ/mol). This parameter quantifies the energy required to convert the liquid into a vapor phase.
Appearance: Trifluoroacetic acid is characterized by its appearance as a colorless liquid. Its transparency is an important feature in various applications.
Odor: Trifluoroacetic acid is known for its pungent smell, which can be readily detected upon exposure. This odor is a distinctive characteristic of the substance.
Also Check – Theoretical Yield Formula
Chemical Properties of Trifluoroacetic Acid
Corrosiveness: Trifluoroacetic acid exhibits corrosive properties when it comes into contact with the skin and eyes. This corrosiveness makes it essential to handle the substance with care and adhere to safety precautions.
Acidity: Due to the presence of three fluorine atoms in its molecular structure, Trifluoroacetic acid displays higher acidity compared to acetic acid. This increased acidity is a consequence of the electron-withdrawing nature of fluorine atoms.
Solubility: Trifluoroacetic acid is soluble in water, indicating its ability to dissolve in aqueous solutions. This solubility is a valuable characteristic for its various applications in aqueous environments.
Electron-Deficiency: The presence of highly electron-withdrawing fluorine atoms in Trifluoroacetic acid renders the carbon atom within the primary functional group highly electron-deficient. This property makes it prone to losing a hydrogen atom.
Also Check – Nucleophile Formula
Uses of Trifluoroacetic Acid
Trifluoroacetic acid finds utility across several domains:
Adhesive and Sealant Chemical: It is employed as an adhesive and sealant chemical in various industrial applications.
Precursor for Fluorinate Compounds: Trifluoroacetic acid serves as a precursor for the production of fluorinated compounds, contributing to the synthesis of a wide range of chemical substances.
Solvent for NMR and Mass Spectroscopy Analysis: TFA is utilized as a solvent in nuclear magnetic resonance (NMR) and mass spectroscopy analyses, aiding in the characterization and identification of chemical compounds.
Also Check – Tyndall Effect Formula
TFA Formula FAQs
What is Trifluoroacetic Acid (TFA)?
What is the Molecular Formula of Trifluoroacetic Acid?
What is the Appearance of Trifluoroacetic Acid?
What is the Boiling Point of Trifluoroacetic Acid?
Is Trifluoroacetic Acid Soluble in Water?










