
Nucleophile Formula is one of the Lewis bases that has an electron pair that is free. The word “nucleophiles” implies this (“nucleus loving”, or “positive-charge loving”). Nucleophile donating electron pairs to electrophiles to form chemical bonds with them, nucleophiles are known as nucleophiles. It is possible for an ion or molecule with a free pair of electrons or a pi bond containing two electrons to behave like a nucleophile. An ion or molecule that contains an electron pair or a pi bond containing two electrons can behave like a nucleophile.
What is Nucleophile ?
Generally, nucleophiles are electron-rich species that donate electron pairs, as discussed earlier. As a result, all nucleophiles are Lewis bases. 'Nucleophile' can be divided into two parts, nucleus and philos. Philos means 'love' in Greek. Hence, nucleophiles are thought of as love-loving species. The following are some terms related to nucleophiles.- Species with a nucleophilic nature are attracted to positively charged nuclei.
- Nucleophilicity refers to a species' nucleophile strength or nucleophilic character.
- An electron-rich nucleophile attacks a positively charged (or partially positively charged) atom in a molecule and bonds with it to replace a leaving group.
Also Read : Malic Acid Formula
The term solvolysis refers to a type of nucleophilic substitution reaction in which the nucleophile is a solvent molecule. Water is a good example of such a nucleophilic solvent and the solvolysis occurs with water.Meaning of Nucleophile
The term 'nucleophile' can be broken down into the parts nucleus and philos. Philos is the Greek word for love. Therefore, nucleophiles are species that love nuclei.Ambident Nucleophiles
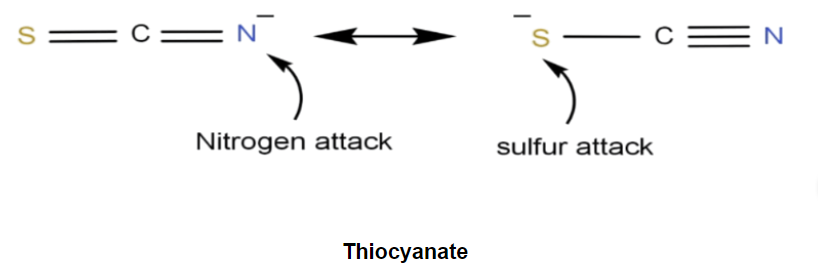
Ambident nucleophiles are nucleophiles that are capable of executing nucleophilic attacks from multiple locations in a molecule (or ion). It is possible for the thiocyanate ion with the chemical formula SCN– to execute nucleophilic attacks from either the sulfur or nitrogen atoms as an example of an ambident nucleophile. This ion often participates in nucleophilic substitution reactions involving alkyl halides, where the following products are formed: alkyl isothiocyanates with the chemical formula R-NCS and alkyl thiocyanates with the chemical formula R-SCN and alkyl isothiocyanates with the same chemical formula R-NCS.Also Check - Monatomic Gases Formula
Therefore, an ambident nucleophile can be thought of as an anionic nucleophile in which the negative charge of the ion is delocalized over two different atoms by resonance effects. Enolate ions often exhibit this quality. The resonance structure of an ambident nucleophile is illustrated below.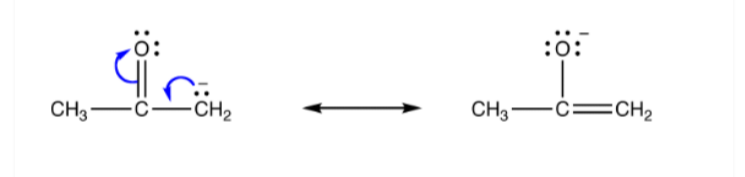
Ambident Nucleophile Examples
An ambient nucleophile is an anionic with weak nucleophilic sites through which it can attack and form a new product. Examples of ambient nucleophiles are cyanide and thiocyanate.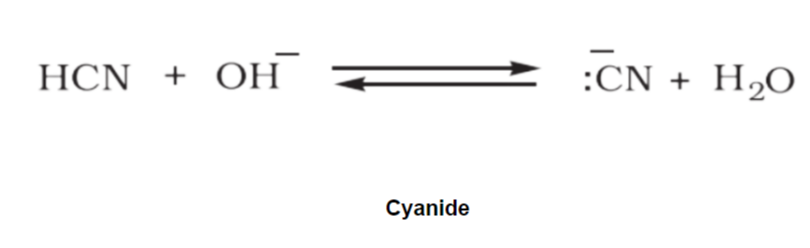
Types of Nucleophiles
Halogens - The diatomic form of a halogen is not nucleophilic. These halogens, however, are excellent nucleophiles when used in anionic form. As an example: diatomic iodine (I2) does not act as a nucleophile in polar, protic solvents, whereas I– is the strongest nucleophile.Also Check - Lead (II) Chloride Formula
Carbon - Grignard Reagents, Organolithium Reagents, and n-butyllithium are some examples of compounds where carbon acts as a nucleophile. Oxygen - The hydroxide ion is a great example of a nucleophile wherein the electron pair is donated by the oxygen atom. Other examples include alcohol and hydrogen peroxide. There are no nucleophilic attacks during intermolecular hydrogen bonding that takes place in many compounds containing oxygen and hydrogen.Also Check - Iron (III) Hydroxide Formula
Sulphur - The large size, the relative ease of its polarization, and the easily accessible lone electron pairs of sulphur make it an ideal nucleophile. Hydrogen sulphide (H2S) is a great example of a nucleophile containing sulphur. Nitrogen - The nucleophilic properties of nitrogen can be found in amines, azides, ammonia, and nitrides. Even amides are known to have nucleophilic properties.Mechanisms of Nucleophilic Substitution
A nucleophilic substitution reaction is influenced not only by the nucleophilicity of the incoming nucleophiles and the leaving capacity of the leaving group, but also by the mechanism through which it occurs. Nucleophilic substitution takes place in two ways: SN2 Mechanism- This mechanism is also known as the substitution nucleophilic bimolecular mechanism.
- While a reaction occurs, the substitution nucleophilic bimolecular mechanism follows second-order kinetics and the rate law for the reaction.
- According to the rate law, the SN2 reaction mechanism depends on the concentration of both the nucleophile and the substrate, increasing the rate of reaction.
- An unimolecular nucleophilic substitution reaction is also known as this mechanism.
- There is no relationship between the nucleophilicity of the incoming nucleophile and the mechanism of the SN1 reaction.
- It depends, however, on the leaving group's capacity to leave.
Things To Remember
- Electrophiles form chemical bonds with nucleophiles by donating electron pairs.
- Nucleophiles include oxygen ions and cyanide ions.
- In ambidextrous nucleophiles, nucleophile attacks are carried out in two or more places in a molecule, forming more than one product.
- A nucleophile attacks a positively charged atom in a molecule and replaces a leaving group by associating with the positively charged species.
- Nucleophilic substitution reactions include solvolysis.
- Nucleophilic substitution mechanisms can be divided into two types, SN1 and SN2.
Nucleophile Formula FAQs
What is a nucleophile in chemistry?
How do nucleophiles differ from electrophiles?
Can you provide examples of common nucleophiles?
What are nucleophilic substitution reactions?
Are all nucleophiles equally reactive?










