
Tin(II) Chloride Formula: Tin(II) chloride, also referred to as stannous chloride, is a white crystalline substance characterized by the chemical formula SnCl 2 . It forms a stable dihydrate, although its aqueous solutions, particularly when subjected to heat, are susceptible to hydrolysis. SnCl 2 finds common usage as a reducing agent under acidic conditions and in electrolytic baths for tin-plating. It's important to distinguish Tin(II) chloride from Tin(IV) chloride, often recognized as stannic chloride (SnCl 4 ).
Also Check – Theoretical Yield Formula
In the gaseous phase, the SnCl 2 molecule exhibits a bent structure due to the presence of a lone pair of electrons. In its solid-state, crystalline SnCl 2 forms chains with chloride bridges. The dihydrate form also consists of three-coordinate entities, with one water molecule coordinated to the tin atom and another to the first water molecule. The "second" water molecule is situated between the layers as the primary component of the molecule stacks into double layers within the crystal lattice
This outlines the structural characteristics of Tin(II) chloride, or stannous chloride.
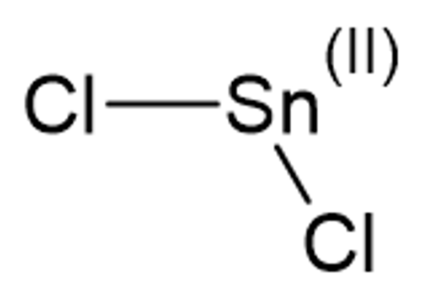
Also Check – Vapor Pressure Formula
Physical Properties of Tin (II) Chloride
- Molecular Formula: SnCl 2
- Molar Mass:
- 189.60 g/mol (anhydrous)
- 225.63 g/mol (dihydrate)
- Appearance: White crystalline solid
- Density:
- 3.95 g/cm³ for the anhydrous solid
- 2.71 g/cm³ for the dihydrate (at 15 °C)
- Melting Point: 246 °C (519 K)
- Boiling Point: 623 °C (896 K)
- Solubility in Water:
- 83.9 g/100 ml at 0 °C
- Undergoes hydrolysis in hot water
Also Check – Dilution Formula
Chemical Properties of Tin (II) Chloride
Tin(II) chloride can dissolve in less than its own mass of water without breaking down. However, upon dilution, hydrolysis occurs, leading to the formation of an insoluble basic salt, as represented by the equation: SnCl 2 + H 2 O ↔ Sn(OH)Cl + HCl Tin(II) chloride acts as a Lewis acid and forms compounds with ligands such as chloride ions, as illustrated by the reaction: SnCl 2 + CsCl → CsSnCl 3 When alkali is added to a solution of SnCl 2 , a white precipitate of hydrated tin(II) oxide initially forms. This precipitate dissolves in excess base, giving rise to stannite salts like sodium stannite, as shown in the reactions: SnCl 2 + 2NaOH → SnO·H 2 O + 2NaCl SnO·H 2 O + NaOH → NaSn(OH) 3 The yellow linear two-coordinate compound Sn(OAr) 2 (where Ar = aryl) is produced when the lithium salt of 4-methyl-2,6-di-tert-butyl-phenol reacts with SnCl 2 in tetrahydrofuran (THF).Also Check – Gibbs Free Energy Formula
Applications of Tin Chloride
Various Applications of Tin Chloride
Effective Reducing Agent: Tin Chloride is employed as a potent reducing agent in various chemical processes.
Pharmaceutical Production: It plays a significant role in the manufacture of pharmaceutical compounds.
Ink Stain Removal: Tin Chloride is utilized for the removal of stubborn ink stains, particularly in cleaning applications.
Lubricating Oil Additive: It is added to lubricating oils to enhance their performance and properties.
Catalyst: Tin Chloride serves as a catalyst in several chemical reactions, facilitating the conversion of substances.
Color Pigment Manufacturing: It is an essential component in the production of color pigments, used in paints, inks, and dyes.
Tin-Plating of Steel: In the metallurgical industry, Tin Chloride is crucial for tin-plating steel surfaces, providing corrosion resistance.
Radionuclide Angiography: In medical diagnostics, Tin Chloride finds application in radionuclide angiography procedures.
Textile Dyeing Mordant: It acts as a mordant in textile dyeing processes, helping to fix dyes onto fabrics.
Polylactic Acid Production: Tin Chloride is a key ingredient in the production of polylactic acid, a biodegradable plastic used in various eco-friendly applications.
Tin Chloride, with its versatile properties and wide-ranging applications, is a valuable chemical compound in various industries. It serves as a powerful reducing agent, contributes to pharmaceutical production, aids in ink stain removal, enhances lubricating oils, acts as a catalyst, and plays a crucial role in the manufacturing of color pigments .
Additionally, it is essential for tin-plating steel, finds use in medical procedures like radionuclide angiography, and acts as a mordant in textile dyeing. Moreover, Tin Chloride plays a pivotal role in the production of eco-friendly plastics, such as polylactic acid. Its multifaceted utility underscores its importance in both industrial and scientific contexts.
Tin(II) Chloride Formula FAQs
What is Tin Chloride?
What is its appearance?
What is the molar mass of Tin Chloride?
What is the melting point of Tin Chloride?
What are some common uses of Tin Chloride?










