
Trichloroacetic acid , also known as TCA, TCAA, or trichloroethanoic acid, is an organic compound with a white crystalline appearance. Its systematic IUPAC name is trichloroacetic acid, and it is a strong acid that serves many purposes in the clinical chemical and biochemistry fields. Its salts and esters are commonly used in cosmetics and referred to as trichloroacetates. The compound was discovered by French Chemist Jean – Baptiste Dumas in 1839 and was later given the alternative name "aceto-caustin." This discovery challenged the established beliefs of Swedish Chemist Jons Jakob Berzelius and his theory of organic radicals and valances. This sparked a lengthy debate between the two chemists, with Dumas being the first to question Berzelius' results from electrochemical research. Before Dumas' discovery, Berzelius' research was widely accepted in the scientific community.
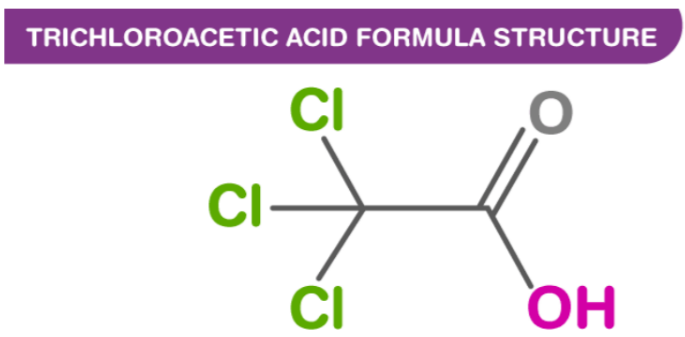
Structure of Trichloroacetic Acid
Carbon atoms bonded to O, OH, and CCl3 groups are sp2 hybridized. Carbon atoms bonded to Cl3, COOH groups are sp3 hybridized.
Uses of Trichloroacetic acid (C 2 HCl 3 O 2 )
The acidic solid nature of trichloroacetic acid makes it suitable for various applications. Some of its applications are listed below.
- In clinical chemistry, it is widely used to prepare formulations.
- Among its applications in biochemistry are precipitating macromolecules, such as proteins, DNA, and RNA. It is also used in chemical peels and tattoo removal.
- It is used in Cosmetics
- Warts are chemoablated with it on the skin.
- It can also be used on genital warts when diluted.
- Skin infections can be treated with it even during pregnancy.
- Until the 1980s, its sodium salt was used as an herbicide.
Also Check – Atomic Mass Formula
Properties of Trichloroacetic Acid
Listed below are the physical and chemical properties of trichloroacetic acid
- The molar mass of this compound is 163.38 g/mol.
- The solid is white or colorless.
- The smell is pungent and sharp.
- It has a density of 1.63 g.cm-3 and a melting point of 58°C.
- The boiling point of this substance is 197°C.
- It dissolves in water
- With a pH of 1.2, it is acidic in nature.
- Blue litmus paper turns red when it is exposed to it.
- The pKa value of this compound is 0.66.
- With a flash point over 93°C, it is a stable compound.
- Water does not react with it.
- Like other strong acids, it is corrosive.
- The dipole moment of this object is 3.23 D.
- Proteins react with it.
Also Check – Elevation of Boiling Point Formula
Preparation of Trichloroacetic Acid
In the presence of a catalyst, chlorine gas reacts with acetic acid to produce it.
CH 3 COOH + 3Cl 2 → CCl 3 COOH + 3HCl
Trichloroacetaldehyde can also be produced by its reaction with oxygen or by its oxidation.
Also Read – Malic Acid Formula
Effects of Trichloroacetic Acid on Health And Environment
As a strong acid, trichloroacetic acid is corrosive. It can injure skin if overused. It can cause serious long-term or temporary injuries to rats when taken in large amounts (5000 mg/kg). It is harmful to the environment as well.
Trichloroacetic Acid Formula FAQs
Q1. What is Trichloroacetic Acid (TCA)?
Q2. How is TCA used in chemical peels?
Q3. Is TCA safe for skin treatments?
Q4. Can TCA be used for other applications besides skincare?
Q5. What precautions should be taken when handling TCA?










