
Chemically, Tungstic Acid Formula is H 2 W O 4 or W O 3 H 2 O . It has a strong acidic flavour and is a white or yellowish powder that dissolves in water. The most widely used commercial form of tungsten is tungsten trioxide, which is hydrated to generate tungstic acid. A variety of tungsten compounds, such as tungsten carbide and tungsten oxide, depend on tungstic acid as a precursor. Additionally, it serves as a catalyst in the synthesis of other organic molecules, including acrylic acid. Additionally, tungstic acid is used in the production of pigments and dyes, as well as in analytical chemistry as a reagent for the measurement of phosphates and arsenates.
Introduction to Tungsten
With an atomic number of 74 and the chemical symbol W, tungsten, sometimes referred to as wolfram, is a chemical element. Only naturally occurring on Earth, the rare metal tungsten is almost usually found in mixtures with other elements. After being identified as a new element in 1781, it was first isolated as a metal in 1783. Two of the element's important ores are scheelite and wolframite, which gives the element its other name. The free element is famous for its toughness since it has the highest melting point of any known element, which is 3,422 °C (6,192 °F; 3,695 K). It also has the greatest boiling point at 5,930 °C (10,706 °F; 6,203 K).Compounds of Tungsten
Tungsten is comparatively chemically inert. However, compounds have been created with the element in oxidation states ranging from 0 to +6. The most typical states are those above +2, particularly +6. Tungsten creates a wide range of complexes in the +4, +5, and +6 stages.Tungsten Carbide
Tungsten carbide (WC), the most significant tungsten compound, is renowned for its hardness (9.5 on the Mohs scale, where the highest, diamond, is 10, according to this scale). Cast iron and the cutting blades of saws and drills are given wear resistance by using it alone or in conjunction with other metals. When tungsten reacts directly with silicon, boron, and nitrogen at high temperatures, it also produces hard, refractory, and chemically inert interstitial compounds with those substances.Also Check - S urface Chemistry Formula
Tungstic Acid Formula
The polymeric substance tungstic acid, which has the chemical formula H 2 W O 4 , is made up of linked chains of tungsten atoms that are each tetrahedrally coordinated to four oxygen atoms. A three-dimensional network of corner-sharing tetrahedra is created by sharing the oxygen atoms with nearby tungsten atoms.Structure of Tungstic Acid Formula
Tungstic acid is a white, amorphous solid powder in its solid form. It dissolves into a 2.5 pH acidic solution when in solution. It is possible to picture the structure of tungstic acid as a polymer of W O 4 tetrahedra that is connected by shared oxygen atoms. Additionally, two hydroxyl (OH) groups are attached to each tungsten atom, adding to the compound's acidic character. [caption id="attachment_15856" align="alignnone" width="300"]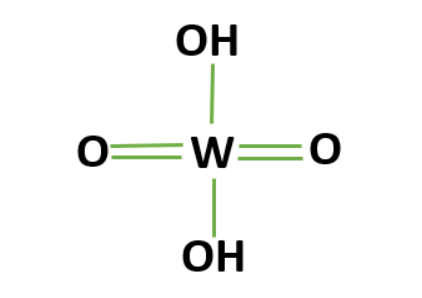 Tungstic Acid Structure[/caption]
Tungstic Acid Structure[/caption]
Preparation of Tungstic Acid
Tungstic acid, often referred to as para-tungstic acid, is a chemical compound having the formula H 2 W O 4 . It may be made through a number of processes, such as the hydrolysis of tungsten hexachloride, the reaction of sodium tungstate with an acid, and the oxidation of tungsten trioxide.Download PDF Tungstic Acid Formula
Procedure:
- 200 mL of distilled water should be used to dissolve 20 grammes of sodium tungstate dihydrate in a glass beaker. Until the sodium tungstate is completely dissolved, stir the mixture.
- The sodium tungstate solution should be mixed with 50 mL of hydrochloric acid. For a few minutes, stir the mixture until the liquid turns hazy.
- Stir the solution continuously for 1–2 hours after heating it to 70–80°C. A yellow precipitate will eventually form from the solution.
- Use filter paper and a funnel to filter the precipitate after allowing the solution to cool to room temperature.
- To get rid of any last-minute contaminants, thoroughly wash the precipitate with distilled water.
- The precipitate must be dried in an oven at 100°C for many hours before it can be ground into a dry, white powder.
Tungstic Acid Formula Applications
Here are a few applications for tungstic acid:- Catalyst: The synthesis of sulfuric acid, a commonly used industrial chemical, uses tungstic acid as a catalyst. Additionally, it functions as a catalyst in the transformation of alkanes into alkenes.
- Tungstic acid is a yellow pigment that is used in ceramics and other materials.
- Due to its capacity to change colour when exposed to an electric current, tungstic acid is utilised in electrochromic devices like smart windows and electrochromic mirrors.
- Tungstic acid is a component of photographic films and X-ray screens because of its high density, which enables it to absorb X-rays and other types of ionising radiation.
- Manufacturing of tungsten carbide: The manufacturing of tungsten carbide, which is used in cutting tools, drilling tools, and other industrial applications, requires tungstic acid as a crucial raw ingredient.
- Chemical analysis: Tin and copper are two elements that are tested for using tungstic acid as a reagent in chemical analysis.
Tungstic Acid Properties
The chemical compound tungstic acid has the colour of yellowish-white and has the formula H 2 W O 4 . It is sometimes referred to as tungstic (VI) acid, wolframic acid, or tungsten acid. The following are some characteristics of tungstic acid:- Physical characteristics: Yellowish-white solid, tungstic acid is a chemical. It has no taste or odour and is insoluble in water.
- Chemical properties: Tungstic acid interacts with a wide range of substances, including metals, non-metals, and organic molecules. It is a powerful oxidising agent. Heat in a reducing environment can convert it to tungsten trioxide.
- Acidic characteristics: Tungstic acid has a pKa of around 1.7, making it a weak acid. It can create the tungstate ion by donating two protons in a solution.
- Thermal stability: Tungstic acid decomposes at higher temperatures, releasing water and producing tungsten trioxide, although it is stable at room temperature.
- Applications: The manufacturing of tungsten metal and alloys, as well as the creation of catalysts and pigments, all include the usage of tungstic acid. Additionally, it serves as an analytical reagent in the chemical sector.
- Toxicity: Although tungstic acid is often thought to have a low toxicity, exposure to high doses can irritate the digestive and respiratory tracts. Large doses of tungstic acid can have negative health consequences when ingested or inhaled.
Tungstic Acid Formula FAQs
What is the formula for Tungstic acid?
What is the purpose of tungstic acid?
Is tungstic acid water soluble?
Is tungsten soluble in acid?
Where does tungstic acid come from?










