ICSE Worksheet for chapter-18 Calorimetry class 10
Worksheet For class 10
This page is prepared by the Academic team of Physics Wallah which consists of ICSE Board Worksheet for Class 10 Physics. Students of Class 10 Physics can get a free Worksheet for Class 10 Physics in PDF format prepared as per the newest syllabus and examination pattern in your schools.
Standard 10 students can practice questions and answers which are given here for Physics in Grade 10 that will help them to improve their knowledge of all important chapters and their topics. Students can also download free pdf of Class 10 Physics Notes prepared by teachers and solve important problems provided here with solutions on daily basis to get more scores in school exams and tests.
If any students need to take the online test to check their concepts or undertstanding then they can visit Physics Quiz for Class 10 .
Summary
- Specific heat capacities and the principle of mixtures(concept of heat and temperature; thermal capacity; c = Q/mΔ T)
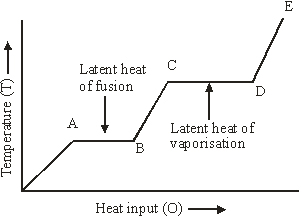
- Latent heat;loss and gain of heat involving a change of state for fusion( heating curve for water; specific latent heat of fusion )
Section 1 - Objective
Q1. 2000 J of energy is needed to heat 1kg of paraffin through 1°C. How much energy is needed to heat 10kg of paraffin through 2°C?
- 4000 J
- 10,000 J
- 20,000 J
- 40,000 J
Q2. The most commonly used thermometric liquid is:
- Water
- Alcohol
- Mercury
- None of these
Q3. Absolute zero corresponds to:
- -273 K
- -273°C
- 273°F
- None of these
Q4. A temperature difference of 30°C in the Fahrenheit scale is:
- 54°F
- 27°F
- 86°F
- -54°F
Q5. With increase in temperature the density of a substance in general:
- Increases
- Decreases
- First increases then decreases
- First decreases then increases
Q6. γ r is coefficient of real expansion of a liquid, γ a is coefficient of apparent expansion of a liquid and γ g is coefficient of cubical expansion of the container then the correct relation is:
- γ r =γ a x γ g
- γ r = γ a ÷ γ g
- γ r = γa– γ g
- γ r = γ a + γ g
Q7. Per °C is unit of:
- α
- β
- γ
- All of these
Q8. Clock’s pendulum is made of invar because:
- It is light in weight
- It is easily available
- Its coefficient of linear expansion is low
- It is cheaply available
Q9. 1 kg of water at 20°C is, mixed with 800g of water at 80°C.Assuming that no heat is lost to the surroundings calculate the final temperature of the mixture:
- 24.44°C
- 46.67°C
- 44.44°C
- 54.44°C
Q10. How much heat would be required to convert 14kg of ice at 0°C into water of 0°C?
- 4704 J
- 4704 kJ
- 336 J
- 336 kJ
Q11. The specific heat capacity of water is 4200 J/kg °C. Calculate the heat capacity of 10kg of water per °C
- 42 J
- 420 J
- 42 kJ
- 420 kJ
Q12. The temperature of a metal coin is increased by 100°C and its diameter increases by 0.15%. Its area increases by nearly:
- 0.15%
- 0.60%
- 0.30%
- 0.0225%
Q13. The change from solid to vapour directly at a constant temperature is called:
- Condensation
- Regelation
- Vaporization
- Sublimation
Q14. The specific latent heat of fusion of water is:
- 80 cal g -1
- 2260 J g -1
- 80 J g -1
- 336 J kg -1
Q15. Expansion in a substance is:
- Directly proportional to rise in the temperature
- Inversely proportional to rise in the temperature
- Independent of rise temperature
- Cannot say
Section 2- Subjective
Q1. Define the following
- Melting point
- Latent heat
- Vaporization
Q2. Distinguish between
- Heat and temperature
- Heat capacity and specific heat capacity
Q3. Calculate the amount of heat required to convert1kg of ice at -10°C into steam at 100°C at normal pressure. Specific heat capacity of ice = 2100 J kg -1 K -1 . Latent heat of fusion of ice =3.36 x 10 6 J kg -1 , specific heat capacity of water = 4200 J kg -1 K -1 and latent heat of vaporization of water =2.25 x 10 6 J kg -1 .
Q4. An iron piece of mass 2kg has a thermal capacity of 966 J/ 0 C.
- How much heat is needed to warm it by 15 0 C?
- What is its specific heat capacity in SI units?
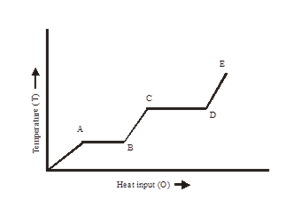
Q5. A source of heat supplies heat at a constant rate to a solid cube. The variation of the temperature of the cube with the heat supplied is shown in the figure given below.
- What does the slope of the part DE represent?
- If CD = 2.5 AB, what does it mean
Solutions
Objective Problems
- D
- C
- B
- C
- B
- D
- D
- C
- B
- B
- C
- C
- D
- A
- A
Subjective Problems
Q3. Q = 3.027 x 10 6 J
Q4. (i) 14490J
(ii) 483 J/Kg/ 0 C
Q5. (i) Slope of the part DE of the graph represents the (ΔT/Q), i.e., the reciprocal of the thermal capacity(Q/ΔT)of the vapour.
(ii) CD = 2.5 AB means the latent heat of vaporisation is 2.5 times the latent heat of fusion.