
SSC Worksheet for Chapter - 2 Chemical Reactions and Their Types Class 8
Worksheets For class 8
Find SSC Worksheet for chapter-2 Chemical Reactions and Their Types class 8
CLASS-8
BOARD: SSC
Chemistry Worksheet - 2
TOPIC: Chemical Reactions and Their Types
For other SCC Worksheet for class 8 Science check out main page of Physics Wallah.
1. When atoms of element ‘A’ and Element ‘B’ react to form molecule ‘D’. It is__________.
(a) Displacement Reactions
(b) Decomposition Reaction
(c) Combination Reaction
(d) Oxidation Reaction
2. Conversion of ice to water is a __________
(a) Physical Change
(b) Chemical Change
(c) Oxidation
(d) Nuclear Change
3.
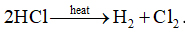 It is an example of
It is an example of
(i) chemical change
(ii) decomposition reaction
(iii) oxidation reaction
(a) (i) & (ii)
(b) only (ii)
(c) (i) & (iii)
(d) All of these
4. Catalyst can ______________ a chemical reaction.
(i) speed up
(ii) de-accelerate
(a) only (i)
(b) only (ii)
(c) none
(d) both (i) & (ii)
5. C + O 2 → CO 2 + Heat is an example of
(i) Oxidation Reaction
(ii) Combination Reaction
(iii) Exothermic Reaction
(iv) Slow Reaction
(a) (i), (iii), (iv)
(b) (i), (ii), (iv)
(c) (i), (ii), (iii)
(d) (i), (ii), (iii), (iv)
6. Chemical reaction between two atoms be best characterised by
(a) transaction electrons
(b) transaction of protons
(c) transaction of nucleons
(d) (a) & (b)
7. Chemical reaction accompanied by reneval of hydrogen is an example of __________reaction.
8. __________ reaction processes with absorption energy in form of heat.
9. Acid and base (alkali) reacts together to from ___________ & ______.
10. Ca( OH ) 2 → CO 2 → (excess) ______H 2 O.
Descriptive:
11. Differentiate between physical and chemical changes.
12. What are the factors on which Rate of reaction depends.
13. Rate of chemical reaction depends on size of the particle. Explain
14. Reduction as reverse process of oxidation. Explain
15. Differentiate between exothermic & Endothermic Reaction.
Solutions: to worksheet-2 Topic- Chemical Reactions and Their Types
1. c
2. a
3. d
4. d
5. c
6. a
7. Oxidation
8. Endothermic
9. Salt and water
10. CaCo 3
11. Temporary changes is shape size or appetence of a substance is thermal are physical change. It is reversible in nature i.e. on an completely regain the initial substance by reversing the conditions. However, the permanent a large in behavior & characteristics of any substance which can not be regained by changing physical conditions are termed as chemical changes.
12. Factors on which rate of reaction relends :
(i) Size of reactant particles
(ii) Temperatire of reactant pastiches.
(iii) Concentration
(iv) Presence of catalysts, promoters or poisons.
13. As the particle size decreases the exposed surface area increases for any given mars of a substance. This leads to greater interaction of reactant particles. Thus leads to increased rest of reaction.
14. Oxidation is addition of oxygen and reduction as removal of oxygen. Similarly, Oxidation is removal of hydrogen & reduction is addition of hydrogen. Thus oxidation & reduction are exactly opposites of each other.
15. Exothermic Reaction: A reaction which proceeds with release of energy (heat) is an exothermic reactions
Exothermic Reaction: A reaction which proceeds with absorption of energy (heat) is an endothermic reaction.









