
Zinc Sulfate Formula : Zinc, denoted by the chemical symbol Zn, is a lustrous bluish-white metal primarily employed for the process of galvanization, which safeguards other metals from corrosion. This essential mineral is naturally found in certain foods and plays a vital role in various aspects of cellular metabolism.
Sulfur, represented by the symbol S, is indispensable for all living organisms. It serves diverse purposes in batteries, fungicides, detergents, fertilizers, fireworks, and matches. Pure sulfur possesses neither taste nor odor and exhibits poor electrical conductivity, while it readily dissolves in water. Classified as a nonmetal, sulfur ranks as the tenth most abundant element in the universe. Notably, it is widely utilized in the form of sulfuric acid for extracting phosphates from ores.
Zinc Sulfate, with the chemical formula ZnSO 4 , is an inorganic compound used as a dietary supplement. It is effective in addressing moderate to severe acne and is composed of both sulfur and zinc. This compound is soluble in water and emits toxic fumes containing zinc oxide and sulfur oxides upon decomposition.Regarding its structure, zinc sulfate consists of a cationic zinc and anionic sulfate entity. In the sulfate anion, sulfur takes the central position, surrounded by four oxygen atoms.
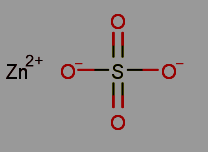 Zinc sulfate is synthesized through several methods, including the reaction between zinc carbonate and sulfuric acid, as expressed by the equation:
4ZnCO
3
+ H
2
SO
4
⇢ ZnSO
4
+ H
2
O + CO
2
Additionally, it forms when zinc reacts with copper sulfate:
Zn + CuSO
4
⇢ ZnSO
4
+ Cu
Furthermore, zinc sulfate can be produced by the reaction between zinc oxide of high purity and sulfuric acid:
ZnO + H
2
SO4 ⇢ ZnSO
4
+ H
2
O
Zinc sulfate is synthesized through several methods, including the reaction between zinc carbonate and sulfuric acid, as expressed by the equation:
4ZnCO
3
+ H
2
SO
4
⇢ ZnSO
4
+ H
2
O + CO
2
Additionally, it forms when zinc reacts with copper sulfate:
Zn + CuSO
4
⇢ ZnSO
4
+ Cu
Furthermore, zinc sulfate can be produced by the reaction between zinc oxide of high purity and sulfuric acid:
ZnO + H
2
SO4 ⇢ ZnSO
4
+ H
2
O
Physical Properties of Zinc Sulfate
Molecular Weight: Zinc Sulfate has a molecular weight of 161.47 g/mol.
Density: The density of Zinc Sulfate is 3.54 g/cm³.
Boiling Point: It has a boiling point of 740°C.
Melting Point: The melting point of Zinc Sulfate is 680°C.
Chemical Properties of Zinc Sulfate
Chemical Formula: The chemical formula of Zinc Sulfate is ZnSO4.
Zinc Sulfate exhibits various chemical reactions, including:
Reaction with Sodium Hydroxide: Zinc Sulfate reacts with sodium hydroxide to yield sodium sulfate and zinc hydroxide:
2 NaOH + ZnSO 4 ⇢ Na 2 SO 4 + Zn(OH) 2Reaction with Potassium Hydroxide: Zinc Sulfate can react with potassium hydroxide to produce potassium sulfate and zinc hydroxide:
2 KOH + ZnSO 4 ⇢ K 2 SO 4 + Zn(OH) 2Reaction with Barium Sulfide: When Zinc Sulfate interacts with barium sulfide, it forms zinc sulfide and barium sulfate:
ZnSO 4 + BaS ⇢ BaSO 4 + ZnSReaction with Sodium Carbonate: Zinc Sulfate reacts with sodium carbonate to generate sodium sulfate and zinc carbonate:
ZnSO 4 + Na 2 CO 3 ⇢ Na 2 SO 4 + ZnCO 3Reaction with Lithium Hydroxide: Zinc Sulfate can also react with lithium hydroxide to produce zinc hydroxide and lithium sulfate:
ZnSO 4 + 2 LiOH ⇢ Zn(OH) 2 + Li 2 SO 4Health Effects of Zinc Sulfate
Exposure to Zinc Sulfate can result in various health effects, including:
- Headaches and vomiting
- Irritation of the respiratory tract
- Nasal and throat irritation
- Coughing and wheezing
Safety Measures for Handling Zinc Sulfate
To safely handle Zinc Sulfate, it is important to:
Read and follow all provided instructions.
- Avoid skin and eye contact.
- Wear protective safety glasses for eye protection.
- Thoroughly wash any contaminated skin after handling.
- Rinse eyes with water if they come into contact with Zinc Sulfate.
- Prevent its release into the environment.
- Keep it out of reach of children and pets.
- Uses of Zinc Sulfate:
Zinc Sulfate Finds Various Applications, Including
Eye Drops: It is used in eye drops.
Electroplating: Zinc Sulfate serves as an electrode in zinc electroplating.
Rayon Production: It is utilized in the production of rayon.
Mineral: Zinc Sulfate is considered a mineral.
Acne Treatment: It is used to alleviate symptoms of mild to moderate acne.
Dietary Supplement: Zinc Sulfate is employed as a dietary supplement to treat zinc deficiency.
Zinc Sulfate Formula FAQs
What is Zinc Sulfate used for?
What are the health effects of Zinc Sulfate exposure?
How can Zinc Sulfate be safely handled?
What are the physical properties of Zinc Sulfate?
What is the chemical formula of Zinc Sulfate?










