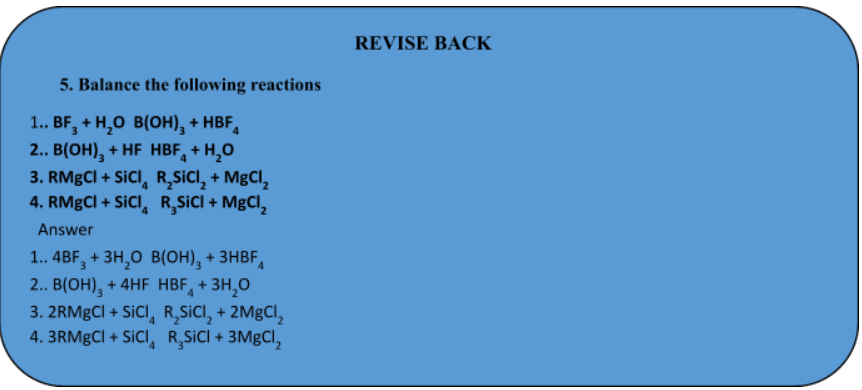
Chemical Equations
Chemical Reaction And Equation of Class 10
A chemical equation is a symbolic representation of an actual chemical change or the short-hand method of representing a chemical reaction in terms of symbols and formulae of the different reactants and products is called a chemical equation.
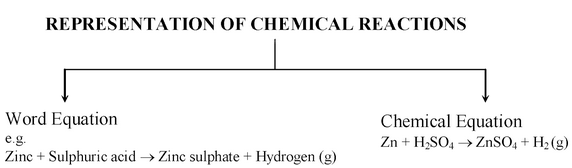
A chemical reaction can be represented in two different ways :
STEPS FOR WRITING A CHEMICAL EQUATION:
Writing of a chemical equation involves the following steps :
- The symbols and formulae of the reactants are written on the left hand side with plus (+) sign between them.
- The symbols and formulae of the products are written on the right hand side with + sign between them.
- An arrow sign (→) is put between the reactants and the products, pointing from reactants towards products.
Word equations: A word equation links together the names of the reactants with those of the products. For example, the word equation, when magnesium ribbon burns in oxygen to form a white powder of magnesium oxide, may be written as follows-
Magnesium + Oxygen → Magnesium oxide
(Reactants) (Product)
Similarly, the word equation for the chemical reaction between granulated zinc and hydrochloric acid may be written as -
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
In a word equation:
- The reactants are written on the left hand side with a plus sign (+) between them.
- The products are written on the right hand side with a plus sign (+) between them.
- An arrow (→) separates the reactants from the products.
- The direction of the arrow head points towards the product.
Symbol equation: A brief representation of a chemical reaction in terms of symbols and formulae of the substances involved is known as a symbol equation.
In a symbol equation, the symbols and formulae of the elements and compounds are written instead of their word names.
For e.g. Burning of magnesium in oxygen to form magnesium oxide may be written as follows:
Mg + O 2 → MgO
BALANCED AND UNBALANCED CHEMICAL EQUATIONS:
Balanced chemical equation:
The equation, in which the number of atoms of each element in the reactants, and the products sides are equal, is called a balanced chemical equation.
The chemical equations are balanced to satisfy the law of conservation of mass in chemical reactions.
|
|
Law of conservation of mass states that, the total mass of the elements present in the products of a chemical reaction has to be equal to the total mass of the elements present in the reactants. |
For example,In a chemical reaction between zinc and dilute sulphuric acid are reactants, giving zinc sulphate and hydrogen as products. The chemical equation can be written as
Zn + H 2 SO 4 → ZnSO 4 + H 2 .
Here the numbers of atoms of each element in the reactant and products sides are equal. i.e.
In reactants In products
No. of Zn atoms 1 1
No. of H atoms 2 2
No. of S atoms 1 1
No. of O atoms 4 4
Hence it is a balanced chemical equation.
Unbalanced chemical equation (skeletal equation):
The equation in which the number of atoms of different elements on the reactants and the product sides are not equal is called an unbalanced chemical equation. The unbalanced chemical equation is also known as skeletal equation.
For example
The burning of aluminium in oxygen to form aluminium oxide can be written as :
Al + O 2 → Al 2 O 3
Here the number of atoms of each element in the reactants and products side is not equal. i.e.
In reactants In products
No. of Al atoms 1 2
No. of O atoms 2 3
Hence it is an unbalanced chemical equation.
Related Topics
BALANCING A CHEMICAL EQUATION:
Balancing of chemical equations may be defined as the process of making the number of different types of elements, on both side of the equations, equal.
The balancing of a chemical equation is done with the help of Hit and Trial method. In this method, the coefficients before the symbols or formulae of the reactants and products are adjusted in such a way that the total number of atoms of each element on both the sides of the arrow head become equal. This balancing is also known as mass balancing because the atoms of elements on both side are equal and their masses will also be equal.
The major steps involved in balancing a chemical equation are as follow –
- Write the chemical equations in the form a word equations. Keep the reactants on the left side and the products on the right side. Separate them by an arrow whose head ( →) points from the reactants towards the product.
- Convert the word equation into the symbol equation by writing the symbols and formulae of all the reactants and product.
- Make the atoms of different elements on both side of the equation equal by suitable method. This is known as balancing of equation.
- Do not change the formulae of the substance while balancing the equation.
- Make the equations more informative if possible.
Let’s consider a reaction --- Iron reacts with water (steam) to form iron (II, III) oxide and liberates hydrogen gas.
The word equation for the reactions is -
Iron + Water → iron (II, III) oxide + Hydrogen
The symbol equation for the same reaction is-
Fe + H 2 O → Fe 3 O 4 + H 2
The balancing of the equations is done are the following steps:
(i) Let us count the number of atoms of all the elements in the reactants and products on both sides of the equation.
Element No. of atoms of reactants No. of atoms of products
(L.H.S.) (R.H.S.)
Fe 1 3
H 2 3
O 2 4
Thus, the numbers of H atoms are equal on both sides. At the same time, the number of Fe and O atoms are not equal.
(ii) On inspection, the number of O atoms in the reactant (H 2 O) is 1 while in the product (Fe 3 O 4 ), it is 4. To balance the atoms, put coefficient 4 before H 2 O on the reactant side. The partially balanced equation may be written as
Fe + 4H 2 O → Fe 3 O 4 + H 2
(iii) In order to equate H atoms, put coefficient 4 before H 2 on the product side, As a result, the H atoms on both sides of the equation become 8 and are thus balanced. The partially balanced equation may now be written as
Fe + 4H 2 O → Fe 3 O 4 + H 2
(iv) In order to balance the Fe atoms, put coefficient 3 before Fe on the reactant side. The equation formed may be written as -
3Fe + 4H 2 O → Fe 3 O 4 + 4H 2
(v) On final inspection, the number of atoms of all the elements on both sides of the equation are equal. Therefore, the equation is balanced.
Writing State Symbols:
The chemical equations or symbol equations which we have enlisted don’t mention the physical states of the reactant and product species involved in the reaction. In order to make the equation more informative, the physical state are also mentioned with the help of certain specific symbols known as state symbols. These symbols are
- (s) for solid state
- (l) for liquid state
- (g) for gaseous state
- (aq) for aqueous solution i.e., solution prepared in water.
Sometimes a gas if evolved in a reaction is shown by the symbol (↑) i.e., by an arrow pointing upwards. Similarly the precipitate, if formed during the reaction, is indicated by the symbol (↓) i.e., by an arrow pointing downwards.
The abbreviation ‘ppt’ is also used to represent the precipitate, if formed.
(i) 2Na(s) + 2H 2 O → 2NaOH (aq) + H 2 (g) or H 2 (↑)
(ii)Ca(OH) 2 (aq) + CO 2 (g) → CaCO 3 (↓) + H2O
(iii) AnNO 3 (aq) + NaCI(aq) → AgCI (↓) + NaNO3 (aq)
Significance of State Symbols:
The state symbols are of most significance for those chemical reactions which are either accompanied by the evolution of heat (exothermic) or by the absorption of heat (endothermic). For example:
2H2 (g) + O 2 (g) → 2H 2 O + 572 kJ
2H 2 (g) + O 2 (g) → 2H 2 O(g) + 44 kJ
Both these reactions are of exothermic nature because heat has been evolved in these. However, actual amounts of heat are different when water is in the liquid state i.e. H2O and when it is in the vapour state.
Specialties of Chemical Equation:
(i) We get the information about the substance which are taking part and formed in the reaction.
(ii) We get the information about the number of molecules of elements or compounds which are either taking part or formed in the chemical reaction.
(iii) We also get the information of weight of the reactants or products.
For example: CaCO 3 → CaO + CO 2
(100gm) (56 gm) (44 gm)
Total weight of the reactants is equal to the total weight of the products because matter is never destroyed. In the above example total weight of calcium carbonate (reactant) is 100 gram and of product is also 100 g (56 gram + 44 gram).
(iv) In a chemical equation if any reactant or product is in gaseous state, then its volume can also be determined. For example in the above reaction volume of carbon dioxide is 22.4 liters.
(v) In a chemical equation with the help of the product we can get information about the valency as well.
For example:
Mg + 2HCl → MgCl 2 + H 2 (↑)
In the above reaction one atom of Mg displaces two atoms of hydrogen, so valency of magnesium is two.