Types Of Chemical Reaction
Chemical Reaction And Equation of Class 10
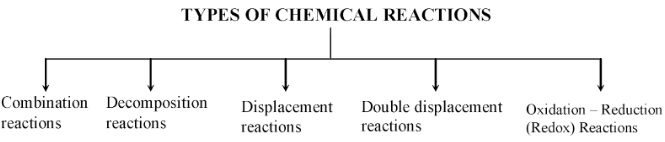
TYPES OF CHEMICAL REACTIONS
COMBINATION REACTIONS:
Those reactions in which two or more substances (reactants) combine together to form a single substance (product) are called the combination reactions.
Ex 1: Formation of water from H 2 (g) and O 2 (g)
2H 2 (g) + O 2 (g) → 2H 2 O(l)
Hydrogen Oxygen Water
In this reaction, two substances hydrogen and oxygen (reactants) combine together to form a single substance i.e. water (product). So it is a combination reaction.
Synthesis reaction: It is a type of addition reaction in which a new substance is formed by the union of its component elements.
For e.g. N 2 + 3H 2 → 2NH 3 (Haber's Process)
Ammonia is synthesised from its components, nitrogen and hydrogen, so it is a synthetic reaction. All synthesis reactions are addition reactions
Other examples of synthesis reactions are:
CaO(s) + H2O(g) → Ca(OH) 2 (aq)
C(s) + O 2 (g) → CO 2 (g)
(i) When two or more compounds combine to form a new compound
For e.g.
NH 3 + HCl → NH 4 Cl
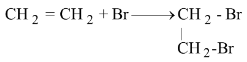
(ii) When an element and a compound combine to form a new compound.
2CO + O 2 → 2CO 2
DECOMPOSITION REACTIONS:
Those reactions in which a single substance (reactant) splits up into two or more simpler substances (products) are known as decomposition reactions.
These reactions are carried out by supplying energy in form of heat, electricity or light which breaks that substance into simpler substances. Thus decomposition reactions are classified as:

- Thermolysis or thermal decomposition reactions (decomposition by heat).
- Electrolysis or electrolytic decomposition reactions (decomposition by electricity)
- Photolysis or photodecomposition reactions (decomposition by light).
Thermal decomposition reactions
|
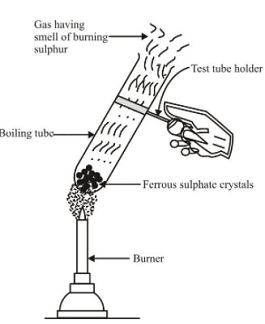
Decomposition reactions which are carried out by heating are called thermal decomposition reactions. Ex 1.Thermal decomposition of ferrous sulphate Experiment : Take a small amount of ferrous sulphate crystals (FeSO 4 . 7H 2 O) in a test tube. These crystals are green in colour. Heat the test tube over a burner. The green colour of crystals first changes to white due to formation of anhydrous ferrous sulphate (FeSO 4 ), as on heating, ferrous sulphate crystals lose the water molecules. On further heating anhydrous ferrous sulphate decomposes to brown solid (which is ferric oxide) along with characteristic smell of burning sulphur. |
Decomposion reaction of ferrous sulphate |
The reactions involved are:

Ex2. Thermal decomposition of Potassium Chlorate: When potassium chlorate is heated in the presence of manganese dioxide as a catalyst, it decomposes to give potassium chloride and oxygen.
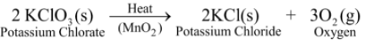
In this reaction, a single substance splits up into two simpler substances on heating. Thus, it is a thermal decomposition reaction.
Ex3.Thermal decomposition of Lead nitrate
Experiment: Take about 2 gm of powdered lead nitrate (colourless) in a dry test tube. Heat the tube over a burner. We observe that brown fumes of nitrogen dioxide are found to evolve and a yellow residue of lead oxide is left behind in the tube. If we put a burning candle over the mouth of test tube, it catches fire and starts burning again. This shows that the oxygen gas is also evolved during this reaction.
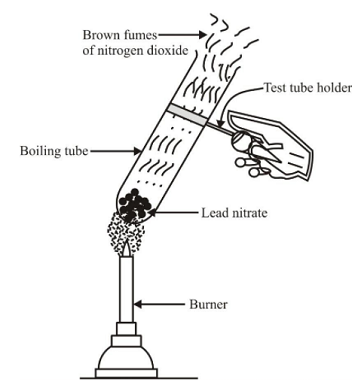
Decomposition reaction of lead nitrate
The reaction involved is :
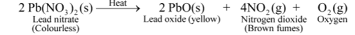
Ex4. Thermal Decomposition of limestone: When calcium carbonate (limestone) is heated, it decomposes to give calcium oxide and carbon dioxide.

Ex5. Thermal decomposition of zinc carbonate : When zinc carbonate is heated, it decomposes to give zinc oxide and carbon dioxide gas.
Related Topics
Electrolytic decomposition Reactions (or electrolysis)
|
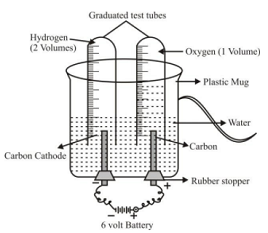
The decomposition reactions which are carried out by using electricity, are called electrolytic decomposition reactions (or electrolysis) Ex 1. Electrolytic decomposition of water or electrolysis of water Experiment: Take a plastic mug and make two holes at the base of mug. Fit two rubber stoppers in these holes, with carbon electrodes. Connect the electrodes to a 6-V battery. Fill the mug with water so that electrodes get immersed in it. Add few drops of dil H2SO4 to make it good conductor of electricity. Fill the two test tubes with water and invert them over the electrodes. Now switch on the battery and keep the apparatus undisturbed for some time. |
Experiment set-up for electrolysis of water |
Observation:
- There is formation of bubbles of two different gases over the electrodes which displace the water in test tubes.
- The gas collected in the test tube covering the cathode is double than that of gas collected in tube covering the anode.
- Keep on passing current till the test tubes are completely filled with respective gases. Now switch off the battery.
Result:
On testing the gases by bringing a burning candle near mouth of tubes, it is found that the gas with double volume, burns with poping sound, so it is hydrogen gas. The other gas with lesser volume, makes the burning candle to glow more, so, it is oxygen gas.
Thus above experiment shows that on supplying electricity, water decomposes into hydrogen and oxygen according to the reaction :
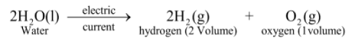
♦ Volume of hydrogen collected is double as that of oxygen because two atoms of hydrogen and one atom of oxygen makeup one molecule of water (H 2 O).
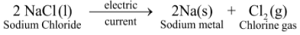
Ex2. Electrolytic decomposition of molten sodium chloride : On passing electric current through molten sodium chloride, it decomposes to give sodium metal and chlorine gas :
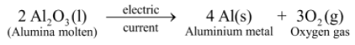
Ex3. Electrolytic decomposition of molten alumina (aluminium oxide) : On passing electric current through molten alumina, it decomposes to give aluminium metal and oxygen gas :
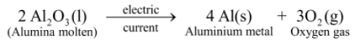
In this reaction, on passing electric current, a single substance i.e. alumina decomposes to give two simpler substances, aluminium metal and oxygen gas, thus it is an example of electrolytic decomposition reaction.
Photo-decomposition reactions (or photolysis):
The decomposition reactions which take place by absorption of light are called the photo-decomposition reactions or photolysis.
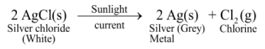
Ex1. Photo-decomposition of silver chloride or Photolysis of silver chloride
Experiment: Take a pinch of silver chloride on a watch glass and keep it in sunlight for some time.
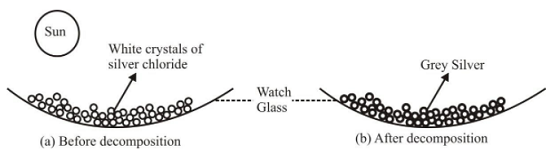
Decomposition of silver chloride in the presence of light
It is observed that white silver chloride turns gray due to formation of silver metal.
Ex2. Photolysis of hydrogen iodide : Hydrogen iodide decomposes in the presence of ultraviolet light into hydrogen and iodine :

Ex3. Photolysis of hydrogen peroxide : In presence of light, hydrogen peroxide decomposes into water and oxygen.
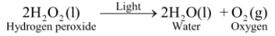
♦ Since hydrogen peroxide decomposes in the presence of light, that is why, hydrogen peroxide is kept in coloured bottles so as to cut off light.
Decomposition reactions are called the opposite of combination reactions
In a decomposition reaction, one substance breaks up into two or more chemical substances, while in a combination reaction two or more substances combine to form one single substance. So these two reactions are called opposite of each other.
Uses of Decomposition Reactions
The decomposition reactions are used in the extraction of metals in the following ways :
(i) Metals are extracted from their molten salts by electrolytic decomposition e.g. sodium from molten sodium chloride and aluminium from alumina (molten aluminium oxide).
(ii) Thermal decomposition reactions form one of the steps in extraction of metals.
For example, Zinc carbonate (the naturally occurring ore of zinc) is first decomposed to give zinc oxide and then reduced to obtain zinc metal i.e.,
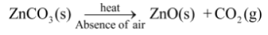

Decomposition Reactions in our body:
The digestion of food in the body is an example of decomposition reaction. When we eat foods like wheat, rice or potatoes, then the starch present in them decomposes to give simple sugars like glucose in the body and proteins decompose to form amino acids :
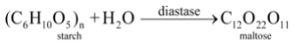
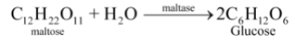

Decomposition Reactions are endothermic reactions:
All decomposition reactions require energy either in form of heat, light or electricity. Hence all decomposition reactions are endothermic (heat absorbing) reactions.